Abstract
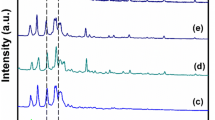
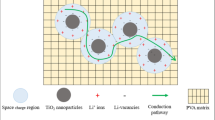
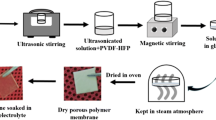
Nanocomposite polymer electrolyte (NCPE) films based on a blend of two polymers poly (ethylene oxide) (PEO) and poly (vinylidene fluoride) (PVDF) complexed with sodium perchlorate (NaClO4) salt and Nano-filler titanium dioxide (TiO2) (i.e., (80 wt% PEO/20 wt% PVDF) + 7.5 wt% NaClO4 + x wt% TiO2 where x = 3, 6, 9, 12, 15, and 18) were prepared and characterized as potential candidates for battery applications. Electrochemical impedance spectroscopy (EIS) has been employed between the frequencies 10 Hz and 4 MHz to investigate electrical, dielectric and electric modulus properties of the prepared NCPE films. Effect of TiO2 Nano-filler concentration on the structural, ionic conductivity, and dielectric relaxation has been studied. The AC conductivity of the NCPE films at high frequencies obeys Jonscher’s power law. The values of DC ionic conductivity calculated by fitting the AC conductivity spectra to the best fit of Joncher’s power law are consistent with the values of DC ionic conductivity calculated from the bulk resistance (Rb) of the NCPE films. The ionic conductivity that depends on temperature follows the Arrhenius rule between the temperatures 298 and 328 K. The maximum ionic conductivity at ambient temperature 8.75 × 10–5 S/cm was obtained for (80 wt% PEO/20 wt% PVDF) + 7.5wt% NaClO4 + 15 wt% TiO2 NCPE film and it is attributed to the decrease in crystallinity. Using Wagner’s polarization technique ionic transport numbers of various NCPE films were measured.











Similar content being viewed by others
References
Y. Yamada, Bull. Chem. Soc. Jpn. (2020). https://doi.org/10.1246/bcsj.20190314
F.J. Sonia, M. Aslam, A. Mukhopadhyay, Carbon (2020). https://doi.org/10.1016/j.carbon.2019.09.026
J.M. Tarascon, M. Armand, Nature (2001). https://doi.org/10.1038/35104644
D. Zhang, R. Li, T. Huang, A. Yu, J. Power Sources (2010). https://doi.org/10.1016/j.jpowsour.2009.08.063
J.A. Lee, J.Y. Lee, M.H. Ryou, G.B. Han, J.N. Lee, D.J. Lee, J.K. Park, Y.M. Lee, J. Solid State Electrochem. (2011). https://doi.org/10.1007/s10008-010-1149-y
V. Palomares, P. Serras, I. Villaluenga, K.B. Hueso, J. Carretero-Gonzalez, T. Rojo, Energy Environ. Sci. (2012). https://doi.org/10.1039/C2EE02781J
H.K. Koduru, Y.G. Marinov, G.B. Hadjichristov, N. Scaramuzza, Solid State Ionics (2019). https://doi.org/10.1016/j.ssi.2019.02.021
L. Wang, Y. Lu, J. Liu, M. Xu, J. Cheng, D. Zhang, J.B. Goodenough, Angew. Chem. (2013). https://doi.org/10.1002/anie.201206854
H.K. Koduru, L. Marino, F. Scarpelli, A.G. Petrov, Y.G. Marinov, G.B. Hadjichristov, M.T. Iliev, N. Scaramuzza, Curr. Appl. Phys. (2017). https://doi.org/10.1016/j.cap.2017.07.012
G. Dave, D.K. Kanchan, Indian J. Pure Appl. Phys. 56, 978 (2018)
K.M. Anilkumar, B. Jinisha, M. Manoj, S. Jayalekshmi, Eur. Polym. J. (2017). https://doi.org/10.1016/j.eurpolymj.2017.02.004
S. Nidhi, R. Patel, Kumar. J Alloys Compd. (2019). https://doi.org/10.1016/j.jallcom.2019.03.089
K. Murata, Electrochim. Acta (1995). https://doi.org/10.1016/0013-4686(95)00160-G
A. Chandra, A. Chandra, K. Thakur, Chin. J. Polym. Sci. (2013). https://doi.org/10.1007/s10118-013-1223-x
H.W. Zhang, P.K. Shen, Chem. Rev. (2012). https://doi.org/10.1021/cr200035s
Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba, R. Kanno, Nat Energy (2016). https://doi.org/10.1038/nenergy.2016.30
J. Li, C. Ma, M. Chi, C. Liang, N.J. Dudney, Adv. Energy Mater. (2015). https://doi.org/10.1002/aenm.201401408
L. Yue, J. Ma, J. Zhang, J. Zhao, S. Dong, Z. Liu, G. Cui, L. Chen, Energy Storage Mater. (2016). https://doi.org/10.1016/j.ensm.2016.07.003
Q. Zhang, K. Liu, F. Ding, X. Liu, Nano Res. (2017). https://doi.org/10.1007/s12274-017-1763-4
M. Gracia, D. Armand, Shanmukaraj, Solid Electrolytes for Advanced Applications (Springer, Cham, 2019), pp. 347–373
F.H.A. El-kader, N.A. Hakeem, R.S. Hafez, A.M. Ismail, J. Inorg. Organomet. Polym. Mater. (2017). https://doi.org/10.1007/s10904-017-0763-x
D.W. Kim, J.K. Park, H.W. Rhee, Solid State Ionics (1996). https://doi.org/10.1016/0167-2738(95)00238-3
P. Dhatarwal, R.J. Sengwa, Indian J. Pure. Appl. Phys. 55, 7 (2017)
S. Das, A. Ghosh, Electrochim. Acta (2015). https://doi.org/10.1016/j.electacta.2015.04.178
Y.L. Nimah, M.Y. Cheng, J.H. Cheng, J. Rick, B.J. Hwang, J. Power Sources (2015). https://doi.org/10.1016/j.jpowsour.2014.11.047
M.A.K.L. Dissanayake, Ionics (2004). https://doi.org/10.1007/BF02382820
S. Choudhary, R.J. Sengwa, J. Inorg. Organomet. Polym. Mater. (2019). https://doi.org/10.1007/s10904-018-1034-1
T.P. Nguyen, Surf. Coat Technol. (2011). https://doi.org/10.1016/j.surfcoat.2011.07.010
A. Hashim, Q. Hadi, J. Inorg. Organomet. Polym. Mater. (2018). https://doi.org/10.1007/s10904-018-0837-4
M. Mohamadi, H. Garmabi, M. Papila, Macromol. Res. (2016). https://doi.org/10.1007/s13233-016-4099-0
K. Xu, Chem. Rev. (2014). https://doi.org/10.1021/cr500003w
E. Quartarone, P. Mustarelli, Chem. Soc. Rev. (2011). https://doi.org/10.1039/C0CS00081G
H.S. Choe, J. Giaccai, M. Alamgir, K.M. Abraham, Elecrochim. Acta (1995). https://doi.org/10.1016/0013-4686(95)00180-M
F. Croce, G.B. Appetecchi, S. Slane, M. Salomon, M. Tavarez, S. Arumugam, Y. Wang, S.G. Reenbaum, Solid State Ionics (1996). https://doi.org/10.1016/0167-2738(96)00137-3
A. Ramanavicius, P. Genys, Y. Oztekin, A. Ramanaviciene, J. Electrochem. Soc. (2014). https://doi.org/10.1149/2.021403jes
A. Ramanavicius, P. Genys, A. Ramanaviciene, Elecrochim. Acta (2014). https://doi.org/10.1016/j.electacta.2014.08.130
A.M. Gaur, D.S. Rana, J. Inorg. Organomet. Polym. Mater. (2019). https://doi.org/10.1007/s10904-019-01126-y
P. Dhatarwal, R.J. Sengwa, Macromol. Res. (2019). https://doi.org/10.1007/s13233-019-7142-0
R.J. Sengwa, S. Choudhary, P. Dhatarwal, J. Mater. Sci. (2019). https://doi.org/10.1007/s10854-019-01587-4
K.K. Ganta, V.R. Jeedi, K.V. Kumar, E.L. Narsaiah, J. Green Eng. 10, 5589 (2020)
N. Angulakshmi, D.J. Yoo, K.S. Nahm, C. Gerbaldi, A.M. Stephan, Ionics (2013). https://doi.org/10.1007/s11581-013-0985-z
P. Prabakaran, R.P. Manimuthu, S. Gurusamy, J. Solid State Electrochem. (2016). https://doi.org/10.1007/s10008-016-3477-z
N.K. Singh, M.L. Verma, M. Minakshi, Bull. Mater. Sci. (2015). https://doi.org/10.1007/s12034-015-0980-2
E. Barsoukov, J.R. Macdonald, Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd edn. (Wiley, Hoboken, NJ, 2015), p. 616
S.B. Aziz, T.J. Woo, M.F.Z. Kadir, H.M. Ahmed, J. Science, Adv. Mater. Devices (2018). https://doi.org/10.1016/j.jsamd.2018.01.002
D. Vanitha, S.A. Bahadur, N. Nallamuthu, S. Athimoolam, A. Manikandan, J. Inorg. Organomet. Polym. Mater. (2016). https://doi.org/10.1007/s10904-016-0468-6
K. Sundaramahalingam, M. Muthuvinayagam, N. Nallamuthu, Polym. Sci. A (2019). https://doi.org/10.1134/S0965545X19050171
S.K.S. Basha, G.S. Sundari, K.V. Kumar, M.C. Rao, Polym. Bull. (2018). https://doi.org/10.1007/s00289-017-2072-5
I.M. Hodge, M.D. Ingram, A.R. West, J. Electroanal. Chem. (1976). https://doi.org/10.1016/S0022-0728(76)80229-X
S.R. Majid, A.K. Arof, Phys. B (2007). https://doi.org/10.1016/j.physb.2006.08.038
R. Manjuladevi, M. Thamilselvan, S. Selvasekarapandian, R. Mangalam, M. Premalatha, S. Monisha, Solid State Ionics (2017). https://doi.org/10.1016/j.ssi.2017.06.002
R.S. Hafez, N.A. Hakeem, A.A. Ward, A.M. Ismail, F.H.A. El-kader, J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01637-z
V.R. Jeedi, E.L. Narsaiah, M. Yalla, R. Swarnalatha, S.N. Reddy, A.S. Chary, Appl. Sci. (2020). https://doi.org/10.1007/s42452-020-03868-8
S. Choudhary, R.J. Sengwa, Indian J. Pure. Appl. Phys. 49, 204 (2011)
A. Karmakar, A. Ghosh, Curr. Appl. Phys. (2012). https://doi.org/10.1016/j.cap.2011.08.017
R.J. Sengwa, P. Dhatarwal, S. Choudhary, Solid State Ionics (2018). https://doi.org/10.1016/j.ssi.2018.07.015
K.K. Ganta, V.R. Jeedi, K.V. Kumar, E.L. Narsaiah, Int. J. Polym. Anal. Charact. (2020). https://doi.org/10.1080/1023666X.2020.1860396
P. Pal, A. Ghosh, Solid State Ionics (2018). https://doi.org/10.1016/j.ssi.2018.02.009
Acknowledgements
The authors sincerely thank the management of B V Raju Institute of Technology (BVRIT-N), Narsapur for continuous support.
Funding
The Jawaharlal Nehru Technological University Hyderabad (JNTUH), India, financially supported this work under grant JNTUH/TEQIP-III/CRS/2019/Physics/04.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganta, K.K., Jeedi, V.R., Katrapally, V. et al. Effect of TiO2 Nano-Filler on Electrical Properties of Na+ Ion Conducting PEO/PVDF Based Blended Polymer Electrolyte. J Inorg Organomet Polym 31, 3430–3440 (2021). https://doi.org/10.1007/s10904-021-01947-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01947-w