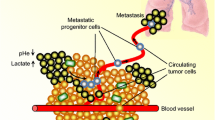
Abstract
Investigating the causes of increased aerobic glycolysis in tumors (Warburg Effect) has gone in and out of fashion many times since it was first described almost a century ago. The field is currently in ascendance due to two factors. Over a million FDG-PET studies have unequivocally identified increased glucose uptake as a hallmark of metastatic cancer in humans. These observations, combined with new molecular insights with HIF-1α and c-myc, have rekindled an interest in this important phenotype. A preponderance of work has been focused on the molecular mechanisms underlying this effect, with the expectation that a mechanistic understanding may lead to novel therapeutic approaches. There is also an implicit assumption that a mechanistic understanding, although fundamentally reductionist, will nonetheless lead to a more profound teleological understanding of the need for altered metabolism in invasive cancers. In this communication, we describe an alternative approach that begins with teleology; i.e. adaptive landscapes and selection pressures that promote emergence of aerobic glycolysis during the somatic evolution of invasive cancer. Mathematical models and empirical observations are used to define the adaptive advantage of aerobic glycolysis that would explain its remarkable prevalence in human cancers. These studies have led to the hypothesis that increased consumption of glucose in metastatic lesions is not used for substantial energy production via Embden-Meyerhoff glycolysis, but rather for production of acid, which gives the cancer cells a competitive advantage for invasion. Alternative hypotheses, wherein the glucose is used for generation of reducing equivalents (NADPH) or anabolic precursors (ribose) are also discussed.
Similar content being viewed by others
References
Anderson ARA (2005) A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol 22:163–186
Baudelet C et al (2004) Physiological noise in murine solid tumors using T2*-weighted gradient echo imaging: a marker for tumor acute hypoxia? Phys Med Biol 49:3389–3411
Beckner ME et al (2005) Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of P13K and gluconeogenesis. Lab Invest 85:1457–1470
Bhujwalla ZM et al (2001) The physiological environment in cancer vascularization, invasion and metastasis. Novartis Found Symp 240:23–38
Bhujwalla ZM et al (2002) Combined vascular and extracellular pH imaging of solid tumors. NMR in Biomed 15(2):114–119
Bos R (2002) Biologic correlates of (18) fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 20:379–387
Braun RD, Lanzel JL, Dewhirst MW (1999) Fourier analysis of fluctuations of oxygen tension and blood flow in R3230Ac tumors and muscle in rats. Am J Physiol 277:t–68
Brown JM, Giaccia AJ (1998) The unique physiology of solid tumors: opportunities (and problems) for cancer theraphy. Cancer Res 58:1408–1416
Brurberg KG, Thuen M, Ruud EB, Rofstad EK (2006) Fluctuations in p02 in irradiated human melanoma xenografts. Radiat Res 165:16–25
Cherk MH et al. (2006) Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med 47:1921–1926
Czernin J, Phelps ME (2002) Positron emission tomography scanning: current and future applications. Ann Rev Med 53:89–112
Dang CV, Semenza GL (1999) Oncogenic alterations of metabolism. Trends Biochem Sci 24:68–72
Dang CV, Lewis BC, Dolde C, Dang G, Shim H (1997) Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembranes 29:345–354
De Jaeger K et al (1998) Heterogeneity of tumor oxygenation: relationship to tumor necrosis, tumor size, and metastasis. Int J Radiat Oncol Biol Phys 42:717–721
Elstrom, RL et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64:3892-3899
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767
Folkman J, Folkman J (2003) Fundamental concepts of the angiogenic process. Curr Mol Med 3:643–651
Fyles A et al (2002) Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. [see comment]. J Clin Oncol 20:680–687
Fyles AW, Milosevic M, Pintilie M, Hill RP (1998) Cervix cancer oxygenation measured following external radiation therapy. Int J Radiat Oncol Biol Phys 42:751–753
Garcia M et al (1996) Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells 14:642–650
Gatenby RA (1998) Mathematical models of tumor-host interactions. Cancer J 11:2–6
Gatenby RA, Gawlinski ET (1996) A reaction-diffusion model of cancer invasion. Cancer Res 56:5745–5753
Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ (2006) Acidmediated tumor invasion: a multidisciplinary study. Cancer Res 66:5216–5223
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Gatenby RA, Vincent TL (2003) An evolutionary model of carcinogenesis. Cancer Res 63:6212–6220
Gillies RJ, Raghunand N, Karczmar GA, Bhujwalla ZM (2002) MR Imaging of the tumor microenvironment. J Magn Reson Imag 15
Green SL, Giaccia AJ (1998) Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy.Cancer J Sci Am 4:218–223
Griffiths JR, McIntyre DJ, Howe FA, Stubbs M (2001) Why are cancers acidic? A carrier-mediated diffusion model for H+ transport in the interstitial fluid. Novartis Found Symp 240:46–62; discussion 62–7, 152–3
Griguer CE et al (2005) Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neuro-Oncol 74:123–133
Guppy M et al (2002) Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J 364:309–315
Haugland HK et al (2002) Expression of hypoxia-inducible factor-1alpha in cervical carcinomas: correlation with tumor oxygenation. Int J Radiat Oncol Biol Phys 53:854–861
Hawkins RA, Phelps ME (1988) PET in clinical oncology. Cancer Metastasis Rev 7:119–142
Helmlinger G, Yuan F, Dellian M, Jain RK (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3:177–182
Hill RP, De Jaeger K, Jang A, Cairns R (2001) pH, hypoxia and metastasis. Novartis Found Symp 240:154–165
Jang A, Hill RP (1997) An examination of the effects of hypoxia, acidosis, and glucose starvation on the expression of metastasis-associated genes in murine tumor cells. Clin Exp Metastasis 15:469–483
Kapp DS, Giaccia AJ (1996) New directions for radiation biology research in cancer of the uterine cervix. J Natl Cancer Inst Monographs 131–139
Kavanagh MC, Sun A, Hu Q, Hill RP (1996) Comparing techniques of measuring tumor hypoxia in different murine tumors: eppendorf pO2 Histograph, [3H]misonidazole binding and paired survival assay. Radiat Res 145:491–500
Kelloff GJ et al (2005) Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res 11:2785–2808
Krogh A (1919a) The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol 52:409–415
Krogh A (1919b) The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol 52:391–408
Langbein S et al (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 94:578–585
Le QT et al (2003) Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. [see comment]. Clin Cancer Res 9:59–67
Montcourrier P et al (1994) Characterization of very acidic phagosomes in breast cancer cells and their association with invasion. J Cell Sci 107:2381–2391
Montcourrier P, Silver I, Farnoud R, Bird I, Rochefort H (1997) Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp. Metastasis 15, 382-392
Naumov GN et al (2006) Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5:1779–1787
Osthus RC et al (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275:21797–21800
Patel AA et al. (2000) Cancer J
Pelicano H et al (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646
Rajendran JG et al (2003) [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nuclear Med Mol Imag 30:695–704
Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ (2005) Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia 7:324–330
Rochefort H, Liaudet E, Garcia M (1996) Alterations and role of human cathepsin D in cancer metastasis. Enzyme Protein 49:106–116
Rofstad EK et al (2006) Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Can Res 66:6699–6707
Rozhin J, Sameni M, Ziegler G, Sloane BF (1994) Pericellular pH affects distribution and secretion of cathepsin B in Malignant Cells. Cancer Res 54:6517–6525
Schlappack OK, Zimmermann A, Hill RP (1991) Glucose starvation and acidosis: effect on experimental metastasic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer 64:663–670
Schornack PA, Gillies RJ (2003) Contributions of cell metabolism and H + diffusion to the acidic pH of tumors. Neoplasia (New York) 5:135–145
Semenza GL (2000) Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 35:71–103
Semenza GL et al (2001) 'The metabolism of tumours': 70 years later. Novartis Found Symp 240:251–260
Semenza GL (2002) Involvement of hypoxia-inducible factor 1 in human cancer. Internal Medicine 41:79–83
Serkova N, Boros LG, Serkova N, Boros LG (2005) Detection of resistance to imatinib by metabolic profiling: clinical and drug development implications. Am J PharmacoGenomics 5:293–302
Smallbone K, Gavaghan DJ, Gatenby RA, Maini PK (2005) The role of acidity in solid tumour growth and invasion. J Theor Biol 235:476–484
Thomlinson RH, Gray LH (1955) The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 9:539–549
Turner GA (1979) Increased release of tumour cells by collagenase at acidic pH: a possible mechanisms for metastasis. Experientia 35:1657–1658
Wykoff CC et al (2001) Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol 158:1011–1019
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH Grants R01 CA 077575 (RJG), and CA 093650 (RAG).
Rights and permissions
About this article
Cite this article
Gillies, R.J., Gatenby, R.A. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis?. J Bioenerg Biomembr 39, 251–257 (2007). https://doi.org/10.1007/s10863-007-9085-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-007-9085-y