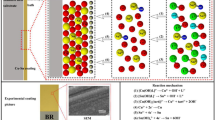
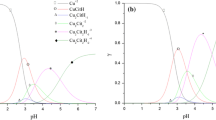
Abstract
The electrodeposition of Cu–Zn–Sn ternary alloys on stainless steel from a cyanide-free alkaline electrolyte containing hydroxyethylidene diphosphonic acid (HEDP) was investigated. Four hydroxyl-containing additives, namely methanol (MET), ethylene glycol (EG), glycerol (GLY), and mannitol (MAN), were added to an HEDP bath, and the effects of the number of hydroxyl groups on the codeposition of Cu–Zn–Sn ternary alloys were compared. Cu–Zn–Sn codeposition occurred at − 0.53 Vvs.Hg|HgO. The hydroxyl-containing additives were beneficial to promote the codeposition of Cu–Zn–Sn, since they can complex with metal ions and act as auxiliary complexing agents. The scanning electron microscopy (SEM) analysis showed that the smallest grain size of 0.1 μm was obtained from the bath containing MAN. In addition to being smaller than the others, these coatings had uniform particle sizes. The energy-dispersive X-ray spectroscopy (EDS) analysis indicated that the composition of the Cu–Zn–Sn coatings obtained from the bath containing MAN was 73.293 wt% Cu, 26.079 wt% Zn, and 0.629 wt% Sn, and these coatings were golden in color. X-ray diffraction (XRD) showed that the Cu–Zn–Sn coatings were crystalline and were composed of a mixture of Cu, Zn, Cu5Zn8, Cu20Sn6, and Cu39Sn11 phases, indicating the formation of Cu–Zn–Sn ternary alloys. These results may provide theoretical guidance for an electrodeposition technology of Cu–Zn–Sn ternary alloys from a new cyanide-free alkaline electrolyte.
Graphic Abstract








Similar content being viewed by others
References
Li J, Ma TT, Wei M, Liu WF, Jiang GS, Zhu CF (2012) The Cu2ZnSnSe4 thin films solar cells synthesized by electrodeposition route. Appl Surf Sci 258:6261–6265
Yang SC, Ho CE, Chang CW, Kao CR (2006) Strong Zn concentration effect on the soldering reactions between Sn-based solders and Cu. J Mater Res 21:2436–2439
Kim YJ, Jo EJ, Kamble AS, Gang MG, Kim JH, Moon JH, Kim JH (2017) Improving the solar cell performance of electrodeposited Cu2ZnSn(S, Se)4 by varying the Cu/(Zn + Sn) ratio. Sol Energy 145:13–19
Clauwaert K, Binnemans K, Matthijs E, Fransaer J (2016) Electrochemical studies of the electrodeposition of copper-zinc-tin alloys from pyrophosphate electrolytes followed by selenization for CZTSe photovoltaic cells. Electrochim Acta 188:344–355
Meng GZ, Sun FL, Wang SJ, Shao YW, Zhang T, Wang FH (2010) Effect of electrodeposition parameters on the hydrogen permeation during Cu–Sn alloy electrodeposition. Electrochim Acta 55:2238–2245
Jung H, Myung NV (2011) Electrodeposition of antimony telluride thin films from acidic nitrate-tartrate baths. Electrochim Acta 56:5611–5615
Zheng JW, Chen HB, Cai W, Qiao L, Ying Y, Li WC, Yu J, Jiang LQ, Che SL (2017) Reaction mechanisms of copper electrodeposition from 1-hydroxyethylidene-1, 1-diphosphonic acid (HEDP) solution on glassy carbon. Mater Sci Eng B 224:18–27
Barbano EP, Oliveira GMD, Carvalho MFD, Carlos IA (2014) Copper-tin electrodeposition from an acid solution containing EDTA added. Surf Coat Technol 240:14–22
Almeida MRHD, Barbano EP, Zacarin MG, Brito MMD, Tulio PC, Carlos IA (2016) Electrodeposition of CuZn films from free-of-cyanide alkaline baths containing EDTA as complexing agent. Surf Coat Technol 287:103–112
Juškėnas R, Karpavičienė V, Pakštas V, Selskis A, Kapočius V (2007) Electrochemical and XRD studies of Cu–Zn coatings electrodeposited in solution with d-mannitol. J Electroanal Chem 602:237–244
Carlos IA, Almeida MRHD (2004) Study of the influence of the polyalcohol sorbitol on the electrodeposition of copper-zinc films from a non-cyanide bath. J Electroanal Chem 562:153–159
Almeida MRHD, Barbano EP, Carvalho MFD, Tulio PC, Carlos IA (2015) Copper-zinc electrodeposition in alkaline-sorbitol medium: Electrochemical studies and structural, morphological and chemical composition characterization. Appl Surf Sci 333:13–22
Rubin W, Oliveira EMD, Carlos IA (2012) Study of the influence of a boric–sorbitol complex on Zn–Mn electrodeposition and on the morphology, chemical composition, and structure of the deposits. J Appl Electrochem 42:11–20
Slupska M, Ozga P (2014) Electrodeposition of Sn–Zn–Cu alloys from citrate solutions. Electrochim Acta 141:149–160
Salhi Y, Cherrouf S, Cherkaoui M, Abdelouahdi K (2016) Electrodeposition of nanostructured Sn–Zn coatings. Appl Surf Sci 367:64–69
Whang TJ, Hsieh MT, Kao YC (2010) Studies of single-step electrodeposition of CuInSe2 thin films with sodium citrate as a complexing agent. Appl Surf Sci 257:1457–1462
Ballesteros JC, Chainet E, Ozil P, Trejo G, Meas Y (2010) Initial stages of the electrocrystallization of copper from non-cyanide alkaline bath containing glycine. J Electroanal Chem 645:94–102
Pewnim N, Roy S (2013) Electrodeposition of tin-rich Cu–Sn alloys from a methanesulfonic acid electrolyte. Electrochim Acta 90:498–506
Whang TJ, Hsieh MT, Kao YC, Lee SJ (2009) A study of electrodeposition of CuInSe2 thin films with triethanolamine as the complexing agent. Appl Surf Sci 255:4600–4605
Rudnik E, Wloch G (2013) Studies on the electrodeposition of tin from acidic chloride–gluconate solutions. Appl Surf Sci 265:839–849
Pecequilo CV, Panossian Z (2010) Study of copper electrodeposition mechanism from a strike alkaline bath prepared with 1-hydroxyethane-1,1-diphosphonic acid through cyclic voltammetry technique. Electrochim Acta 55:3870–3875
Leon PFJD, Albano EV, Salvarezza RC (2002) Interface dynamics for copper electrodeposition: the role of organic additives in the growth mode. Phys Rev E 66:042601
Ding LF, Liu F, Cheng J, Niu YL (2018) Effects of four N-based additives on imitation gold plating. J Appl Electrochem 48:175–185
Tang A, Li Z, Wang F, Dou M, Liu J, Ji J, Song Y (2017) Electrodeposition mechanism of quaternary compounds Cu2 ZnSnS4: Effect of the additives. Appl Surf Sci 427:267–275
Lallemand F, Ricq L, Wery M, Bercot P, Pagetti J (2004) The influence of organic additives on the electrodeposition of iron-group metals and binary alloy from sulfate electrolyte. Appl Surf Sci 228:326–333
Silva FLG, Lago DCBD, Elia ED, Senna LF (2010) Electrodeposition of Cu–Zn alloy coatings from citrate baths containing benzotriazole and cysteine as additives. J Appl Electrochem 40:2013–2022
Carvalho MFD, Barbano EP, Carlos IA (2013) Influence of disodium ethylenediaminetetraacetate on zinc electrodeposition process and on the morphology, chemical composition and structure of the electrodeposits. Electrochim Acta 109:798–808
Zhao X, Zhang JJ, Qu JH (2015) Photoelectrocatalytic oxidation of Cu-cyanides and Cu-EDTA at TiO2 nanotube electrode. Electrochim Acta 180:129–137
Ballesteros JC, Díaz-Arista P, Meas Y, Ortega R, Trejo G (2007) Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochim Acta 52:3686–3696
Khelladi MR, Mentar L, Azizi A, Kadirgan F, Schmerber G, Dinia A (2012) Nucleation, growth and properties of Co nanostructures electrodeposited on n-Si (1 1 1). Appl Surf Sci 258:3907–3912
Joint Committee on Powder Diffraction Standards, JCPDS (2000), Powder diffraction file- PDF-2, Database Sets 1–49. ICDD, Pennsylvania, (CDROM).
He XC, Shen HL, Wang W, Pi JH, Hao Y, Shi XB (2013) Synthesis of Cu2ZnSnS4 films from co-electrodeposited Cu–Zn–Sn precursors and their microstructural and optical properties. Appl Surf Sci 282:765–769
Hsieh YT, Tsai RW, Su CJ, Sun IW (2014) Electrodeposition of CuZn from chlorozincate ionic liquid: from hollow tubes to segmented nanowires. J Phys Chem C 118:22347–22355
Gancarz T, Pstrus J, Berent K (2016) Interfacial reactions of Zn–Al alloys with Na addition on Cu substrate during spreading test and after aging treatments. J Mater Eng Perform 25:3366–3374
Carvalho MFD, Barbano EP, Carlos IA (2015) Electrodeposition of copper-tin-zinc ternary alloys from disodium ethylenediaminetetraacetate bath. Surf Coat Technol 262:111–122
El-Ashram T, Shalaby RM (2005) Effect of rapid solidification and small additions of Zn and Bi on the structure and properties of Sn–Cu eutectic alloy. J Electron Mater 34:212–215
Yan JJ, Chang LC, Lu CW, Dow WP (2013) Effects of organic acids on through-hole filling by copper electroplating. Electrochim Acta 109:1–12
Schah-Mohammedi P, Shenderovich IG, Detering C, Limbach HH, Tolstoy PM, Smirnov SN, Golubev NS (2000) Hydrogen/Deuterium-Isotope effects on NMR chemical shifts and symmetry of homoconjugated hydrogen-bonded ions in polar solution. J Am Chem Soc 122:12878–12879
Palanki MSS, Akiyama H, Campochiaro P, Cao J, Chow CP (2008) Development of prodrug 4-chloro-3-(5-methyl-3-{[4-(2-pyrrolidin-1-ylethoxy) phenyl ] amino}-1, 2, 4-benzotriazin-7-yl) phenyl benzoate (TG100801): a topically administered therapeutic candidate in clinical trials for the treatment of age-related macular degeneration. J Med Chem 51:1546–1559
Rode S, Henninot C, Vallières C, Matlosz M (2004) Complexation chemistry in copper plating from citrate baths. J Electrochem Soc 151:405–411
Zhang J, Liu AM, Ren XF, Zhang JQ, Yang PX, An MZ (2014) Electrodeposit copper from alkaline cyanide-free baths containing 5, 5ˊ-dimethylhydantoin and citrate as complexing agents. RSC Adv 4:38012–38026
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC51604180), the Applied Basic Research Programs of Science and Technology Department of Shanxi Province (201701D221036), and Cultivate Scientific Research Excellence Programs of Higher Education Institutions in Shanxi (CSREP2019KJ038).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, J., Ding, L., Li, Q. et al. Effect of hydroxyl-containing additives on the codeposition of Cu–Zn–Sn alloys. J Appl Electrochem 50, 475–488 (2020). https://doi.org/10.1007/s10800-020-01405-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-020-01405-4