Abstract
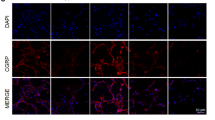
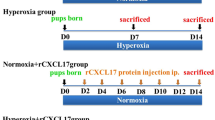
As a stem cell of alveolar epithelium, the physiological status of alveolar epithelium type II cells (AECII) after hyperoxia exposure is closely related to the occurrence of hyperoxia-induced lung injury and the restoration of normal morphological function of damaged alveolar epithelium. However, the relevant mechanisms involved are not very clear. Therefore, this study aimed to explore the effect of calcitonin gene-related peptide (CGRP) on AECII exposed to hyperoxia and its potential mechanisms. The AECII viability was detected using MTT assay. The malondialdehyde (MDA) level and superoxide dismutase (SOD) activity were detected by spectrophotometry. The transdifferentiation capacity of AECII was evaluated by flow cytometry. The expression levels of Notch1, Hes, HERP, and AECII markers were detected using immunohistochemistry and/or RT-qPCR or immunofluorescence. ELISA was used for the determination of inflammatory markers. The results showed that CGRP significantly promoted cell viability, and markedly suppressed hyperoxia-induced transdifferentiation of AECII; these biological alterations were coincided with decreased MDA level, increased SOD activity, and activated Notch signaling pathway (upregulated expression levels of Notch1, Hes, and HERP). Notably, the in vitro effects of CGRP on Notch signaling pathway were further investigated in animal model, and the HE staining results showed that CGRP reduced in vivo oxidative injury and inflammation in hyperoxia-treated AECII through the promotion of structural and functional regeneration, accompanied by elevated Notch1 expression and activated Notch signaling cascade as shown by immunohistochemistry and QPCR, respectively. Immunohistochemistry of APQ-5 and SPC indicated that CGRP reversed the transdifferentiation of AECIIs in vivo. Our current results were consistent across both in vitro and in vivo settings, and provide a new direction for the prevention and treatment of bronchopulmonary dysplasia (BPD).





Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed during this study are included in this submitted article.
References
Jason, G., and J.P. Kinsella. 2011. Pathogenesis and treatment of bronchopulmonary dysplasia. Current Opinion in Pediatrics 23 (3): 305–313.
Hernandez-Ronquillo, L., J.F. Tellez-Zenteno, N. Weder-Cisneros, V. Salinas-Ramirez, J.A. Zapata-Pallagi, and O. da Silva. 2004. Risk factors for the development of bronchopulmonary dysplasia: A case-control study. Archives of medical research 35 (6): 549–553. https://doi.org/10.1016/j.arcmed.2004.07.001.
Konigshoff, M., and O. Eickelberg. 2010. WNT signaling in lung disease: A failure or a regeneration signal?. American journal of respiratory cell and molecular biology 42 (1): 21–31. https://doi.org/10.1165/rcmb.2008-0485TR.
Fehrenbach, H. 2001. Alveolar epithelial type II cell: Defender of the alveolus revisited. Respiratory research 2 (1): 33–46. https://doi.org/10.1186/rr36.
Kawanami, Y., Y. Morimoto, H. Kim, T. Nakamura, K. Machida, T. Kido, E. Asonuma, K. Yatera, C. Yoshii, and M. Kido. 2009. Calcitonin gene-related peptide stimulates proliferation of alveolar epithelial cells. Respiratory research 10: 8. https://doi.org/10.1186/1465-9921-10-8.
Smillie, S.J., and S.D. Brain. 2011. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 45 (2): 93–104. https://doi.org/10.1016/j.npep.2010.12.002.
Dakhama, A., J.W. Park, C. Taube, M. El Gazzar, T. Kodama, N. Miyahara, K. Takeda, A. Kanehiro, A. Balhorn, A. Joetham, J.E. Loader, G.L. Larsen, and E.W. Gelfand. 2005. Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection. American journal of physiology Lung cellular and molecular physiology 288 (4): L761-770. https://doi.org/10.1152/ajplung.00143.2004.
Wu, D.M., P.A. van Zwieten, and H.N. Doods. 2001. Effects of calcitonin gene-related peptide and BIBN4096BS on myocardial ischemia in anesthetized rats. Acta pharmacologica Sinica 22 (7): 588–594.
Li, Y.J., Z.S. Xiao, C.F. Peng, and H.W. Deng. 1996. Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. European journal of pharmacology 311 (2–3): 163–167. https://doi.org/10.1016/0014-2999(96)00426-8.
Fu, H. M., Feng Xu, C. J. Liu, F. W. Kuang, Bo Huang, Fang Fang, and Y. Q. Fu. 2008. [Influence of 60% oxygen inhalation on type II alveolar epithelial cells and protective role of calcitonin gene-related peptide from their damage induced in premature rat]. Zhongguo wei zhong bing ji jiu yi xue = Chinese critical care medicine = Zhongguo weizhongbing jijiuyixue 20 (10): 578–581.
Fu, H., T. Zhang, R. Huang, Z. Yang, C. Liu, M. Li, F. Fang, and F. Xu. 2017. Calcitonin gene-related peptide protects type II alveolar epithelial cells from hyperoxia-induced DNA damage and cell death. Experimental and Therapeutic Medicine 13 (4): 1279–1284. https://doi.org/10.3892/etm.2017.4132.
Xu, K., N. Moghal, and S.E. Egan. 2012. Notch signaling in lung development and disease. Advances in experimental medicine and biology 727: 89–98. https://doi.org/10.1007/978-1-4614-0899-4_7.
Zaher, T.E., E.J. Miller, D.M. Morrow, M. Javdan, and L.L. Mantell. 2007. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free radical biology & medicine 42 (7): 897–908. https://doi.org/10.1016/j.freeradbiomed.2007.01.021.
Gore, A., M. Muralidhar, M.G. Espey, K. Degenhardt, and L.L. Mantell. 2010. Hyperoxia sensing: From molecular mechanisms to significance in disease. Journal of immunotoxicology 7 (4): 239–254. https://doi.org/10.3109/1547691x.2010.492254.
Tatsuya, I., K. Larry, and H. Yasuo. 2003. HES and HERP families: Multiple effectors of the Notch signaling pathway. Journal of Cellular Physiology 194 (3): 237–255.
Boonyatecha, N., N. Sangphech, W. Wongchana, P. Kueanjinda, and T. Palaga. 2012. Involvement of Notch signaling pathway in regulating IL-12 expression via c-Rel in activated macrophages. Molecular Immunology 51 (3–4): 255–262.
Grabher, C., H. von Boehmer, and A.T. Look. 2006. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nature Reviews Cancer 6 (5): 347–359. https://doi.org/10.1038/nrc1880.
Grosveld, G.C. 2009. Gamma-secretase inhibitors: Notch so bad. Nature Medicine 15 (1): 20–21. https://doi.org/10.1038/nm0109-20.
Zhou, M., Z.L. Cui, X.J. Guo, L.P. Ren, M. Yang, Z.W. Fan, R.C. Han, and W.G. Xu. 2015. Blockade of Notch signalling by gamma-secretase inhibitor in lung T cells of asthmatic mice affects T cell differentiation and pulmonary inflammation. Inflammation 38 (3): 1281–1288. https://doi.org/10.1007/s10753-014-0098-5.
Bai, Yu-xin, Fang Fang, Jia-ling Jiang, and Feng Xu. 2017. Extrinsic calcitonin gene-related peptide inhibits hyperoxia-induced alveolar epithelial type II cells apoptosis, oxidative stress, and reactive oxygen species (ROS) production by enhancing Notch 1 and homocysteine-induced endoplasmic reticulum protein (HERP) expression. Medical science monitor: international medical journal of experimental and clinical research 23: 5774–5782. https://doi.org/10.12659/msm.904549.
Mao, C., J.Q. Yuan, Y.B. Lv, X. Gao, Z.X. Yin, V.B. Kraus, J.S. Luo, C.L. Chei, D.B. Matchar, Y. Zeng, and X.M. Shi. 2019. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: A community-based cohort study. BMC Geriatrics 19 (1): 104. https://doi.org/10.1186/s12877-019-1109-z.
Hong, Wang, Chang Liwen, and Li Wenbin. 2005. Temporal expression of Notch in preterm rat lungs exposed to hyperoxia. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban 25(2): 159–161, 165.
Knabe, C., B. Kraska, C. Koch, U. Gross, H. Zreiqat, and M. Stiller. 2006. A method for immunohistochemical detection of osteogenic markers in undecalcified bone sections. Biotechnic & histochemistry: Official publication of the Biological Stain Commission 81 (1): 31–39. https://doi.org/10.1080/10520290600725474.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, Calif) 25 (4): 402–408. https://doi.org/10.1006/meth.2001.1262.
Huang, B., H. Fu, M. Yang, F. Fang, F. Kuang, and F. Xu. 2009. Neuropeptide substance P attenuates hyperoxia-induced oxidative stress injury in type II alveolar epithelial cells via suppressing the activation of JNK pathway. Lung 187 (6): 421–426. https://doi.org/10.1007/s00408-009-9177-z.
Ghadiali, S., and Y. Huang. 2011. Role of airway recruitment and derecruitment in lung injury. Critical reviews in biomedical engineering 39 (4): 297–317.
Miyake, Yasunobu, Hitomi Kaise, Kyo-ichi Isono, Haruhiko Koseki, Kenji Kohno, and Masato Tanaka. 2007. Protective role of macrophages in noninflammatory lung injury caused by selective ablation of alveolar epithelial type II cells. Journal of immunology (Baltimore, Md : 1950) 178(8): 5001–5009. https://doi.org/10.4049/jimmunol.178.8.5001.
Dang, H.X., J. Li, C. Liu, Y. Fu, F. Zhou, L. Tang, L. Li, and F. Xu. 2017. CGRP attenuates hyperoxia-induced oxidative stress-related injury to alveolar epithelial type II cells via the activation of the Sonic hedgehog pathway. International journal of molecular medicine 40 (1): 209–216. https://doi.org/10.3892/ijmm.2017.3002.
Li, Y., J. Wang, H.Y. He, L.J. Ma, J. Zeng, G.C. Deng, X. Liu, J.F. Engelhardt, and Y. Wang. 2011. Immunohistochemical demonstration of airway epithelial cell markers of guinea pig. Tissue & cell 43 (5): 283–290. https://doi.org/10.1016/j.tice.2011.05.003.
Feng, G., Q. Wang, X. Xu, Z. Liu, Z. Li, and G. Liu. 2011. The protective effects of calcitonin gene-related peptide on gastric mucosa injury of gastric ischemia reperfusion in rats. Immunopharmacology and immunotoxicology 33 (1): 84–89. https://doi.org/10.3109/08923971003762774.
Song, S., N. Liu, W. Liu, R. Shi, K.J. Guo, and Y.F. Liu. 2009. The effect of pretreatment with calcitonin gene-related peptide on attenuation of liver ischemia and reperfusion injury due to oxygen free radicals and apoptosis. Hepato-gastroenterology 56 (96): 1724–1729.
Ayala, A., M.F. Munoz, and S. Arguelles. 2014. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity 2014: 360438. https://doi.org/10.1155/2014/360438.
Folz, R.J., A.M. Abushamaa, and H.B. Suliman. 1999. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. The Journal of clinical investigation 103 (7): 1055–1066. https://doi.org/10.1172/jci3816.
Hou, A., J. Fu, H. Yang, Y. Zhu, Y. Pan, S. Xu, and X. Xue. 2015. Hyperoxia stimulates the transdifferentiation of type II alveolar epithelial cells in newborn rats. American journal of physiology Lung cellular and molecular physiology 308 (9): L861-872. https://doi.org/10.1152/ajplung.00099.2014.
Lu, H.Y., G.B. Shao, W.B. Li, and H. Wang. 2011. Effects of hyperoxia on transdifferentiation of primary cultured type II alveolar epithelial cells from premature rats. In vitro cellular & developmental biology Animal 47 (1): 64–72. https://doi.org/10.1007/s11626-010-9360-9.
Akram, K.M., N. Patel, M.A. Spiteri, and N.R. Forsyth. 2016. Lung regeneration: Endogenous and exogenous stem cell mediated therapeutic approaches. International Journal of Molecular Sciences 17: 128. https://doi.org/10.3390/ijms17010128.
Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: Cell fate control and signal integration in development. Science (New York, NY) 284 (5415): 770–776. https://doi.org/10.1126/science.284.5415.770.
Sang, L., J.M. Roberts, and H.A. Coller. 2010. Hijacking HES1: How tumors co-opt the anti-differentiation strategies of quiescent cells. Trends in molecular medicine 16 (1): 17–26. https://doi.org/10.1016/j.molmed.2009.11.001.
Iso, T., V. Sartorelli, G. Chung, T. Shichinohe, L. Kedes, and Y. Hamamori. 2001. HERP, a new primary target of Notch regulated by ligand binding. Molecular and Cellular Biology 21 (17): 6071–6079. https://doi.org/10.1128/mcb.21.17.6071-6079.2001.
He, Y., and L. Zou. 2019. Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Experimental and Therapeutic Medicine 18 (3): 1884–1890. https://doi.org/10.3892/etm.2019.7765.
Kadesch, T. 2004. Notch signaling: The demise of elegant simplicity. Current opinion in genetics & development 14 (5): 506–512. https://doi.org/10.1016/j.gde.2004.07.007.
Jiang, S., S. Han, J. Chen, X. Li, and H. Che. 2017. Inhibition effect of blunting Notch signaling on food allergy through improving TH1/TH2 balance in mice. Annals of Allergy, Asthma & Immunology 118 (1): 94–102. https://doi.org/10.1016/j.anai.2016.10.024.
Song, S.G., H.Y. Yu, Y.W. Ma, F. Zhang, and X.Y. Xu. 2016. Inhibition on Numb/Notch signal pathway enhances radiosensitivity of lung cancer cell line H358. Tumour Biology 37 (10): 13705–13719. https://doi.org/10.1007/s13277-016-5134-7.
Funding
This work was supported by the Natural Science Foundation of Shenzhen Science and Technology Innovation Commission in 2020 [grant number JCYJ20190813141207091].
Author information
Authors and Affiliations
Contributions
JD wrote the manuscript, obtained grants from the national funding agency, and designed the work. SW designed the work and supervised the study to be implemented smoothly. XZ and ZT contributed to the analysis and interpretation of data.
Corresponding author
Ethics declarations
Ethics Approval
The study protocol was approved by the Animal Care and Use Committee of University of South China. All animal experiments were performed with the minimum number of animals, and care was taken to minimize any suffering to the animals with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, J., Wang, SH., Zheng, XM. et al. Calcitonin Gene-Related Peptide Attenuates Hyperoxia-Induced Oxidative Damage in Alveolar Epithelial Type II Cells Through Regulating Viability and Transdifferentiation. Inflammation 45, 863–875 (2022). https://doi.org/10.1007/s10753-021-01591-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01591-z