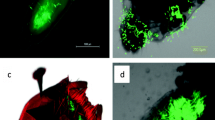
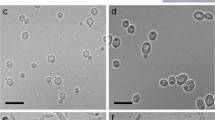
Abstract
Some phytophagous insects have been known to inoculate certain fungi on plant substrates. In many cases of such insect–fungi relationships it has been considered that fungi contribute to insects by decomposing lignin or polysaccharides, and that the insects feed on the decomposition products or fungi themselves. Females of the leaf-rolling weevil in the genus Euops (Attelabidae) store spores of symbiotic fungi in the mycangia and inoculate them on leaf rolls. To determine the effect of mycangial fungi on larval nutrition in E. lespedezae, the nutritional value was compared between leaves with and without mycangial fungi. Two Penicillium species were isolated from the mycangia. These mycangial fungi showed little effect on the decomposition of lignin and polysaccharides, and showed little effect on enhancement of soluble sugars within leaves. Thus, the mutualism between Euops and its mycangial fungi contrasts with the mainly nutritional mutualisms between wood-infesting insects (termites, bark/ambrosia beetles, and wood wasps) and lignin/polysaccharide-decomposing fungi.


Similar content being viewed by others
References
Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ (2002) The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA 23:14887–14892
Abe T, Higashi M (1991) Cellulose centered perspective on terrestrial community structure. Oikos 60:127–133
Abril AB, Bucher EH (2002) Evidence that the fungus cultured by leaf-cutting ants does not metabolize cellulose. Ecol Lett 5:325–328
Bacci M Jr, Anversa MM, Pagnocca FC (1995) Cellulose degradation by Leucocoprinus gongylophorus, the fungus cultured by the leaf-cutting ant Atta sexdens rubropilosa. Antonie von Leeuwenhoek 67:385–386
Barras SJ, Hodges JD (1969) Carbohydrates in inner bark of Pinus taeda as affected by Dendroctonus frontalis and associated microorganisms. Can Entomol 101:489–493
Batra LR (1979) Insect–fungus symbiosis. Allenheld & Osmun, Montclair, NJ
Batra LR, Batra SWT (1979) Termite–fungus mutualism. In: Batra LR (ed) Insect–fungus symbiosis. Allenheld & Osmun, Montclair, NJ, pp 77–116
Bridges JR (1983) Mycangial fungi of Dendroctonous frontalis (Coleoptera: Scolytidae) and their relationship to beetle population trends. Environ Entomol 12:858–861
Bridges JR, Perry TJ (1985) Effects of mycangial fungi on gallery construction and distribution of bluestain in southern pine beetle-infested pine bolts. J Entomol Sci 20(2):271–275
D’Ettorre P, Mora P, Dibangou V, Rouland C, Errard C (2002) The role of the symbiotic fungus in the digestive metabolism of two species of fungus-growing ants. J Comp Physiol B 172:169–176
Domsch KH, Gams W, Anderson T-H (1993) Compendium of soil fungi, vol 1. IHW-Verlag, Eching
Evans HC (1989) Mycopathogens of insects of epigeal and aerial habitats. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds) Insect–fungus interactions. Academic Press, London, pp 205–238
Gardes M, Bruns TD (1993) ITS primer with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rust. Mol Ecol 21:113–118
Haack RA, Slansky F Jr (1987) Nutritional ecology of wood-feeding Coleoptera, Lepidoptera, and Hymenoptera. In: Slansky F Jr, Rodriguez JG (eds) Nutritional ecology of insects, mites, spiders, and related invertebrates. Wiley, New York, pp 449–486
Hochuli DF (1996) The ecology of plant/insect interactions: implications of digestive strategy for feeding by phytophagous insects. Oikos 75:133–141
Hölldbler B, Wilson EO (1990) The ants. Belknap, Cambridge
Hyodo F, Inoue T, Azuma J-I, Tayasu I, Abe T (2000) Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol Biochem 32:653–658
Iwamoto S, Tokumasu S, Suyama Y, Kakishima M (2002) Molecular phylogeny of four selected species of the strictly anamorphic genus Thysanophora using nuclear ribosomal DNA sequences. Mycoscience 43:169–180
Kendrick B (2000) The fifth kingdom, 3rd edn. Focus Publishing, USA
Kukor JJ, Martin MM (1983) Acquisition of digestive enzymes by siricid woodwasps from their fungal symbiont. Science 220:1161–1163
Lee KE, Wood TG (1971) Termites and soil. Academic Press, London
Littledyke M, Cherrett JM (1976) Direct ingestion of plant sap from cut leaves by the leaf-cutting ants Atta cephalotes (L.) and Acromyrmex octospinosus (Reich) (Formicidae, Attini). Bull Entomol Res 66:205–217
Madden JL (1988) Sirex in Australasia. In: Berryman AA (ed) Dynamics of forest insect populations: patterns, causes, implications. Plenum, New York, pp 407–429
Martin MM (1991) The evolution of cellulose digestion in insects. Philos Trans R Soc Lond B 333:281–288
Martin MM, Martin JS (1978) Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis: the role of acquired digestive enzymes. Science 199:1453–1455
Martin MM, Weber NA (1969) The cellulose-utilizing capability of the fungus cultured by the Attine ant Atta colombica tonsipes. Ann Entomol Soc Am 62:1386–1387
Martin T, Oliveira L, Garcia P (2005) Larval mortality factors of Spodoptera littoralis in the Azores. BioControl 50:761–770
Maynard AL, Loosli BS, Harold FH, Warner RG (1979) Animal nutrition. McGrow-Hill Book Company, New York
Miura K, Kudo M (1970) An agar-medium for aquatic hyphomycetes. Trans Mycol Soc Jpn 11:116–118 (in Japanese)
Morgan FD (1968) Bionomics of siricidae. Annu Rev Entomol 13:239–256
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Osono T (2002) Phyllosphere fungi on leaf litter of Fagus crenata: occurrence, colonization, and succession. Can J Bot 80:460–469
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
Peterson SW (2000) Phylogenetic analysis of Penicillium species based on ITS and lsu-rDNA nucleotide sequences. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Hardwood, Amsterdam, pp 163–178
Quinlan RJ, Cherrett JM (1979) The role of fungus in the diet of the leaf-cutting ant. Ecol Entomol 4:151–160
Richard F-J, Mora P, Errard C, Rouland C (2005) Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J Comp Physiol B 175:297–303
Riedel A (2002) Taxonomy, phylogeny, and zoogeography of the weevil genus Euops (Insecta: Coleoptera: Curcurionoidea) in the Papuan region. PhD thesis, Ludwig Maximilians University, Munich
Rohlfs M, Obmann B, Peterson R (2005) Competition with filamentous fungi and its implication for a gregarious lifestyle in insects living on ephemeral resources. Ecol Entomol 30:556–563
Sakurai K (1985) An attelabid weevil (Euops splendida) cultivates fungi. J Ethol 3:151–156
Sawada Y, Morimoto K (1986) The mycetangia and the mode of the fungus transmission in the weevil genus Euops (Coleoptera: Attelabidae). Sci Bull Fac Agr Kyusyu Univ 40(4):197–205 (in Japanese with English summary)
Schoonhoven LM, Jermy T, van Loon JJA (1997) Insect–plant biology. Chapman & Hall, London
Silva A, Bacci M Jr, Siqueira CG, Bueno OC, Pagnocca FC, Hebling MJA (2003) Survival of Atta sexdens workers on different food sources. J Insect Physiol 49:307–313
Siqueira CG, Bacci M Jr, Pagnocca FC, Bueno OC, Hebling MJA (1998) Metabolism of plant polysaccharides by Leucoagaricus gongylophorus, the symbiotic fungun of the leaf-cutting ant Atta sexdens L. Appl Environ Microb 64:4820–4822
Suzuki K, Uehara C (1997) Cradle structure and formation process in the subfamilies Apoderinae and Attelabinae (Coleoptera, Attelabidae) from Japan. Bull Hoshizaki Green Found 1:99–204
Takabe N (2004) Host use of Euops splendidus and dynamics of its mutualistic fungi through development of the beetle. Master’s thesis, Nagoya University (in Japanese)
Talmadge KW, Keegstra K, Bauer WD, Albersheim P (1973) The structure of plant cell wall. Plant Physiol 51:158–173
Watanabe H, Noda H, Tokuda G, Lo N (1998) A cellulase gene of termite origin. Nature 394:330–331
Wheeler Q, Blackwell M (1984) Fungus–insect relationships. Columbia University Press, New York
Wilding N, Collins NM, Hammond PM, Webber JF (1989) Insect–fungus interactions. Academic Press, London
Yamaji K, Fukushi Y, Hashidoko Y, Yoshida T, Tahara S (1999) Characterization of antifungal metabolites produced by Penicillium species isolated from seeds of Picea glehnii. J Chem Ecol 25:1643–1645
Acknowledgements
We thank Naoki Takabe and Hisashi Kajimura for providing important information regarding the mycangial fungi of Euops; Seiji Tokumasu for identification of the fungi; and Takuo Hishi for helpful advice on the statistical analyses. This study is supported by JSPS Research Fellowships for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, C., Fukasawa, Y., Hirose, D. et al. Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol 22, 711–722 (2008). https://doi.org/10.1007/s10682-007-9196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-007-9196-2