Abstract
The chicken genome was the third vertebrate to be sequenced. To date, its sequence and feature annotations are used as the reference for avian models in genome sequencing projects developed on birds and other Sauropsida species, and in genetic studies of domesticated birds of economic and evolutionary biology interest. Therefore, an accurate description of this genome model is important to a wide number of scientists. Here, we review the location and features of a very basic element, the centromeres of chromosomes in the galGal5 genome model. Centromeres are elements that are not determined by their DNA sequence but by their epigenetic status, in particular by the accumulation of the histone-like protein CENP-A. Comparison of data from several public sources (primarily marker probes flanking centromeres using fluorescent in situ hybridization done on giant lampbrush chromosomes and CENP-A ChIP-seq datasets) with galGal5 annotations revealed that centromeres are likely inappropriately mapped in 9 of the 16 galGal5 chromosome models in which they are described. Analysis of karyology data confirmed that the location of the main CENP-A peaks in chromosomes is the best means of locating the centromeres in 25 galGal5 chromosome models, the majority of which (16) are fully sequenced and assembled. This data re-analysis reaffirms that several sources of information should be examined to produce accurate genome annotations, particularly for basic structures such as centromeres that are epigenetically determined.

Similar content being viewed by others
Abbreviations
- CENP-A:
-
centromere protein A
- ChIP:
-
chromatine immuno-precipitation
- DNA:
-
deoxyribonucleic acid
- G:
-
Giga
- bp:
-
base pairs
- seq:
-
sequencing
References
Abyzov A, Urban AE, Snyder M, Gerstien M (2011) CNVnator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Gen Res 21:974–984. https://doi.org/10.1101/gr.114876.110
Aird D, Ross MG, Chen WS, Danielsson M, Fennell T, Russ C, Jaffe DB, Nusbaum C, Gnirke A (2011) Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol 12:R18. https://doi.org/10.1186/gb-2011-12-2-r18
Aldrup-Macdonald ME, Sullivan BA (2014) The past, present, and future of human centromere genomics. Genes (Basel) 5:33–50
Andreozzi L, Federico C, Motta S, Saccone S, Sazanova AL, Sazanov AA, Smirnov AF, Galkina SA, Lukina NA, Rodionov AV, Carels N, Bernardi G (2001) Compositional mapping of chicken chromosomes and identification of the gene-richest regions. Chromosom Res 9:521–532
Benjamini Y, Speed TP (2012) Summarizing and correcting the GC content bias in high-throughput sequencing. Nucleic Acids Res 40(10):e72. https://doi.org/10.1093/nar/gks001
Bersani F, Lee E, Kharchenko PV, Xu AW, Liu M, Xega K, MacKenzie OC, Brannigan BW, Wittner BS, Jung H, Ramaswamy S, Park PJ, Maheswaran S, Ting DT, Haber DA (2015) Pericentromeric satellite repeat expansions through RNA-derived DNA intermediates in cancer. Proc Natl Acad Sci U S A 112:15148–15153. https://doi.org/10.1073/pnas.1518008112
Bitgood JJ, Shoffner RN, Otis JS, Wang N (1982) Recombinant inversion chromosomes in phenotypically normal chickens. Science 215:409–411
Chaisson MJP, Wilson RK, Eichler EE (2015) Genetic variation and the de novo assembly of human genomes. Nat Rev Genet 16:627–640. https://doi.org/10.1038/nrg3933
Copenhaver GP (2003) Using Arabidopsis to understand centromere function: progress and prospects. Chromosom Res 11:255–262
Costantini M, Di Filippo M, Auletta F, Bernardi G (2007) Isochore pattern and gene distribution in the chicken genome. Gene 400:9–15. https://doi.org/10.1016/j.gene.2007.05.025
Dabney J, Meyer M (2012) Length and GC-biases during sequencing library amplification: a comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. BioTechniques 52:87–94. https://doi.org/10.2144/000113809
Eden FC, Hendrick JP, Gottlieb SS (1978) Homology of single copy and repeated sequences in chicken, duck, japanese quail, and ostrich DNA. Biochemistry 17:5113–5121
Fechheimer NS (1990) Chromosomes of chickens. Adv Vet Sci Comp Med 34:169–207
Federico C, Cantarella CD, Scavo C, Saccone S, Bed'Hom B, Bernardi G (2005) Avian genomes: different karyotypes but a similar distribution of the GC-richest chromosome regions at interphase. Chromosom Res 13:785–793. https://doi.org/10.1007/s10577-005-1012-7
Guizard S, Piégu B, Arensburger P, Guillou F, Bigot Y (2016) Deep landscape update of dispersed and tandem repeats in the genome model of the red jungle fowl, Gallus gallus, using a series of de novo investigating tools. BMC Genomics 17:659. https://doi.org/10.1186/s12864-016-3015-5
Gurevich A, Saveliev V, Vyahhi N, Tesler G (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. https://doi.org/10.1093/bioinformatics/btt086
Henikoff S, Ahmad K, Malik HS (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293:1098–1102. https://doi.org/10.1126/science.1062939
Hori T, Kagawa N, Toyoda A, Fujiyama A, Misu S, Monma N, Makino F, Ikeo K, Fukagawa T (2017) Constitutive centromere-associated network controls centromere drift in vertebrate cells. J Cell Biol 216:101–113. https://doi.org/10.1083/jcb.201605001
Hori T, Shang WH, Toyoda A, Misu S, Monma N, Ikeo K, Molina O, Vargiu G, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2014) Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev Cell 29:740–149. https://doi.org/10.1016/j.devcel.2014.05.001
International Chicken Genome Sequencing Consortium (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432:695–716. https://doi.org/10.1038/nature03154
Kapusta A, Suh A (2017) Evolution of bird genomes—a transposon’s-eye view. Ann N Y Acad Sci 1389:164–185. https://doi.org/10.1111/nyas.13295
Kapusta A, Suh A, Feschotte C (2017) Dynamics of genome size evolution in birds and mammals. Proc Natl Acad Sci U S A 114:E1460–E1469. https://doi.org/10.1073/pnas.1616702114
Khiste N, Ilie L (2015) LASER: large genome ASsembly EvaluatoR. BMC Res Notes 8:709. https://doi.org/10.1186/s13104-015-1682-y
Khost DE, Eickbush DG, Larracuente AM (2017) Single-molecule sequencing resolves the detailed structure of complex satellite DNA loci in Drosophila melanogaster. Genome Res 27:709–721. https://doi.org/10.1101/gr.213512.116
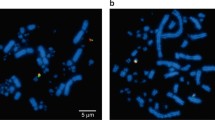
Krasikova A, Deryusheva S, Galkina S, Kurganova A, Evteev A, Gaginskaya E (2006) On the positions of centromeres in chicken lampbrush chromosomes. Chromosom Res 14:777–789. https://doi.org/10.1007/s10577-006-1085-y
Krasikova A, Fukagawa T, Zlotina A (2012) High-resolution mapping and transcriptional activity analysis of chicken centromere sequences on giant lampbrush chromosomes. Chromosom Res 20:995–1008. https://doi.org/10.1007/s10577-012-9321-0
Kretschmer R, Ferguson-Smith MA, de Oliveira EHC (2018) Karyotype evolution in birds: from conventional staining to chromosome painting. Genes (Basel) 9. doi:https://doi.org/10.3390/genes9040181
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with bowtie 2. Nat Methods 9:357–359. https://doi.org/10.1038/nmeth.1923
Matzke MA, Varga F, Berger H, Schernthaner J, Schweizer D, Mayr B, Matzke AJ (1990) A 41-42 bp tandemly repeated sequence isolated from nuclear envelopes of chicken erythrocytes is located predominantly on microchromosomes. Chromosoma 99:131–137
Mello CV, Lovell PV (2018) Avian genomics lends insights into endocrine function in birds. Gen Comp Endocrinol 256:123–129. https://doi.org/10.1016/j.ygcen.2017.05.023
Miller MM, Robinson CM, Abernathy J, Goto RM, Hamilton MK, Zhou H, Delany ME (2014) Mapping genes to chicken microchromosome 16 and discovery of olfactory and scavenger receptor genes near the major histocompatibility complex. J Hered 105:203–215. https://doi.org/10.1093/jhered/est091
Nakamura K, Oshima T, Morimoto T, Ikeda S, Yoshikawa H, Shiwa Y, Ishikawa S, Linak MC, Hirai A, Takahashi H, Altaf-Ul-Amin M, Ogasawara N, Kanaya S (2011) Sequence-specific error profile of Illumina sequencers. Nucleic Acids Res 39:e90. https://doi.org/10.1093/nar/gkr344
Oyola SO, Otto TD, Gu Y, Maslen G, Manske M, Campino S, Turner DJ, Macinnis B, Kwiatkowski DP, Swerdlow HP, Quail MA (2012) Optimizing Illumina next-generation sequencing library preparation for extremely AT-biased genomes. BMC Genomics 13:1. https://doi.org/10.1186/1471-2164-13-1
Peona V, Weissensteiner MH, Suh A (2018) How complete are ‘complete’ genome assemblies?—an avian perspective. Mol Ecol Resour. https://doi.org/10.1111/1755-0998.12933
Plohl M, Meštrović N, Mravinac B (2014) Centromere identity from the DNA point of view. Chromosoma 123:313–325. https://doi.org/10.1007/s00412-014-0462-0
Ross MG, Russ C, Costello M, Hollinger A, Lennon NJ, Hegarty R, Nusbaum C, Jaffe DB (2013) Characterizing and measuring bias in sequence data. Genome Biol 14:R51. https://doi.org/10.1186/gb-2013-14-5-r51
Scott KC, Sullivan BA (2014) Neocentromeres: a place for everything and everything in its place. Trends Genet 30:66–74. https://doi.org/10.1016/j.tig.2013.11.003
Sedlazeck FJ, Lee H, Darby CA, Schatz MC (2018) Piercing the dark matter: bioinformatics of long-range sequencing and mapping. Nat Rev Genet 19:329–346. https://doi.org/10.1038/s41576-018-0003-4
Seroussi E, Pitel F, Leroux S, Morisson M, Bornelöv S, Miyara S, Yosefi S, Cogburn LA, Burt DW, Anderson L, Friedman-Einat M (2017) Mapping of leptin and its syntenic genes to chicken chromosome 1p. BMC Genet 18:77. https://doi.org/10.1186/s12863-017-0587-2
Shang WH, Hori T, Martins NM, Toyoda A, Misu S, Monma N, Hiratani I, Maeshima K, Ikeo K, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2013) Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell 24:635–648. https://doi.org/10.1016/j.devcel.2013.02.009
Shang WH, Hori T, Toyoda A, Kato J, Popendorf K, Sakakibara Y, Fujiyama A, Fukagawa T (2010) Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res 20:1219–1228. https://doi.org/10.1101/gr.106245.110
Tanne A, Muniz LR, Puzio-Kuter A, Leonova KI, Gudkov AV, Ting DT, Monasson R, Cocco S, Levine AJ, Bhardwaj N, Greenbaum BD (2015) Distinguishing the immunostimulatory properties of noncoding RNAs expressed in cancer cells. Proc Natl Acad Sci U S A 112:15154–15159. https://doi.org/10.1073/pnas.1517584112
Thomma BPHJ, Seidl MF, Shi-Kunne X, Cook DE, Bolton MD, van Kan JAL, Faino L (2016) Mind the gap; seven reasons to close fragmented genome assemblies. Fungal Genet Biol 90:24–30. https://doi.org/10.1016/j.fgb.2015.08.010
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192. https://doi.org/10.1093/bib/bbs01
Wang X, Li J, Leung FC (2002) Partially inverted tandem repeat isolated from pericentric region of chicken chromosome 8. Chromosom Res 10:73–82
Warren WC, Hillier LW, Tomlinson C, Minx P, Kremitzki M, Graves T, Markovic C, Bouk N, Pruitt KD, Thibaud-Nissen F et al (2017) A new chicken genome assembly provides insight into avian genome structure. G3 1:109–117. https://doi.org/10.1534/g3.116.035923
Weissensteiner MH, Pang AWC, Bunikis I, Höijer I, Vinnere-Petterson O, Suh A, Wolf JBW (2017) Combination of short-read, long-read and optical mapping assemblies reveals large-scale tandem repeat arrays with population genetic implications. Genome Res 27:697–708. https://doi.org/10.1101/gr.215095.116
Wolf JBW, Ellegren H (2017) Making sense of genomic islands of differentiation in light of speciation. Nat Rev Genet 18:87–100. https://doi.org/10.1038/nrg.2016.133
Zhang G, Li C, Li Q, Li B, Larkin DM, Lee C, Storz JF, Antunes A, Greenwold MJ, Meredith RW et al (2014) Comparative genomics reveals insights into avian genome evolution and adaptation. Science 346:1311–1320. https://doi.org/10.1126/science.1251385
Zhang J, Li C, Zhou Q, Zhang G (2015) Improving the ostrich genome assembly using optical mapping data. Gigascience 4:24. https://doi.org/10.1186/s13742-015-0062-9
Zlotina A, Galkina S, Krasikova A, Crooijmans RP, Groenen MA, Gaginskaya E, Deryusheva S (2010) Precise centromere positioning on chicken chromosome 3. Cytogenet Genome Res 129:310–313. https://doi.org/10.1159/000314923
Zlotina A, Galkina S, Krasikova A, Crooijmans RP, Groenen MA, Gaginskaya E, Deryusheva S (2012) Centromere positions in chicken and Japanese quail chromosomes: de novo centromere formation versus pericentric inversions. Chromosom Res 20:1017–1032. https://doi.org/10.1007/s10577-012-9319-7
Acknowledgements
Peter Arensburger holds a senior researcher fellowship from the STUDIUM.
Funding
This work was funded by the Project Région Centre AviGeS, the C.N.R.S., the I.N.R.A., the Groupement de Recherche CNRS 2157, and the Ministère de l’Education Nationale, de la Recherche et de la Technologie.
Author information
Authors and Affiliations
Contributions
YB and BP conceived the study and analyzed data. YB, PA and FG wrote the paper.
Corresponding author
Additional information
Responsible Editor: Beth A. Sullivan
Electronic supplementary material
ESM 1
(XLSX 38 kb)
Rights and permissions
About this article
Cite this article
Piégu, B., Arensburger, P., Guillou, F. et al. But where did the centromeres go in the chicken genome models?. Chromosome Res 26, 297–306 (2018). https://doi.org/10.1007/s10577-018-9585-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-018-9585-0