Abstract
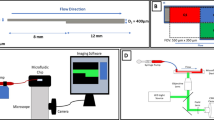
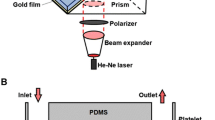
We report a novel device to analyze cell-surface interactions under controlled fluid-shear conditions on well-characterised protein surfaces. Its performance is demonstrated by studying platelets interacting with immobilised von Willebrand Factor at arterial vascular shear rates using just 200 μL of whole human blood per assay. The device’s parallel-plate flow chamber, with 0.1 mm2 cross sectional area and height-to-width ratio of 1:40, provides uniform, well-defined shear rates along the chip surface with negligible vertical wall effects on the fluid flow profile while minimizing sample volumetric flow. A coating process was demonstrated by ellipsometry, atomic force microscopy, and fluorescent immunostaining to provide reproducible, homogeneous, uniform protein layers over the 0.7 cm2 cell-surface interaction area. Customized image processing quantifies dynamic cellular surface coverage vs. time throughout the whole-blood-flow assay for a given drug treatment or disease state. This device can track the dose response of anti-platelet drugs, is suitable for point-of-care diagnostics, and is designed for adaptation to mass manufacture.







Similar content being viewed by others
Notes
Some protein is expected to adsorb onto the PMMA surface; however, this should not affect the results, as the depth of focus, which is centered on the fluid-contacting surface of the glass bottom plate, is only 20 μm (objective working distance = 190 μm, slide thickness = 170 μm); furthermore, the flow conditions are such that any platelet that interacts with a side wall or the top plate will not reach the bottom plate within the microscope’s field of view.
References
C.H. Ahn, J.W. Choi, G. Beaucage, J.H. Nevin, J.B. Lee, A. Puntambekar, J.Y. Lee, Disposable Smart lab on a chip for point-of- care clinical diagnostics. Proc IEEE 92, 154–173 (2004)
K. Almalah, J. McGuire, R. Sproull, A Macroscopic Model for the Single-Component Protein Adsorption-Isotherm. J Colloid Interface Sci 170, 261–268 (1995)
H. Becker, L.E. Locascio, Polymer microfluidic devices. Talanta 56, 267–287 (2002)
T. Boone, Z.H. Fan, H. Hooper, A.J. Ricco, H.D. Tan, S. Williams, Plastic advances microfluidic devices. Anal Chem 74, 78A–86A (2002)
D.C. Brown, R.S. Larson, Improvements to parallel plate flow chambers to reduce reagent and cellular requirements. BMC Immunol 2, 1–7 (2001)
H. Bruus, Theoretical Microfluidics (Oxford University Press, Oxford, 2007)
W.C. Chang, L.P. Lee, D. Liepmann, Biomimetic technique for adhesion-based collection and separation of cells in a microfluidic channel. Lab Chip 5, 64–73 (2005)
R. Daw, J. Finkelstein, Lab on a chip. Nature 442, 367–367 (2006)
P.G. De Groot, J. Sixma Jan, Perfusion Chambers, Chapter 32, in Platelets, ed. by A.D. Michelson, 2nd edn. (Elsevier/Academic Press, San Diego, 2007), pp. 575–585
J. El-Ali, P.K. Sorger, K.F. Jensen, Cells on chips. Nature 442, 403–411 (2006)
H. Elwing, Protein absorption and ellipsometry in biomaterial research. Biomaterials 19, 397–406 (1998)
C. Fillafer, G. Ratzinger, J. Neumann, Z. Guttenberg, S. Dissauer, I. Lichtscheidl, M. Wirth, F. Gabor, M. Schneider, An acoustically driven biochip—impact of flow on the cell-association of targeted drug carriers. Lab Chip 9(19), 2782–2788 (2009)
J. Garcia-Cordero, A.J. Ricco, Lab on a Chip (General Philosophy) in Encyclopedia of Micro- and Nanofluidics, 962–969 (Springer, Berlin, 2008)
E. Gutierrez, B. Petrich, S. Shattil, M. Ginsberg, A. Groisman, A. Kasirer-Friede, Microfluidic devices for studies of shear dependent platelet adhesion. Lab Chip 8, 1486–1495 (2008)
P. Harrison, Platelet function analysis. Blood Rev 19, 111–123 (2005)
P. Harrison, Assessment of platelet function in the laboratory. Hamostaseologie 29, 25–31 (2009)
A.S. Kantak, B.K. Gale, Y. Lvov, S.A. Jones, Platelet function analyzer: Shear activation of platelets in microchannels. Biomed Microdevices 5, 207–215 (2003)
M.H. Kroll, J.D. Hellums, L.V. McIntire, A.I. Schafer, J.L. Moake, Platelets and shear stress. Blood 88, 1525–1541 (1996)
B. Lincoln, A.J. Ricco, N.J. Kent, L. Basabe-Desmonts, L.P. Lee, B.D. MacCraith, D. Kenny, G. Meade, Integrated system for the study of fluid shear-mediated platelet interactions with Von Willebrand Factor protein surfaces using microliter volumes of whole blood. doi:10.1016/j.ab.2010.05.030
H. Lu, L.Y. Koo, W.C.M. Wang, D.A. Lauffenburger, L.G. Griffith, K.F. Jensen, Microfluidic shear devices for quantitative analysis of cell adhesion. Anal Chem 76, 5257–5264 (2004)
A.D. Michelson, Platelets 2nd Ed (Academic Press, USA, 2006), pp. 145–158
N.A. Mody, O. Lomakin, T.A. Doggett, T.G. Diacovo, M.R. King, Mechanics of transient platelet adhesion to von Willebrand factor under flow. Biophys J 88, 1432–1443 (2005)
K. Nakanishi, T. Sakiyama, K. Imamura, On the adsorption of proteins on solid surfaces, a common but very complicated phenomenon. J Biosci Bioeng 91, 233–244 (2001)
NI Vision Concepts Manual. Ed. Corporation, N.I. (2000–2005). http://www.ni.com/pdf/manuals/372916e.pdf
N. Saeidi, E. Sander, J. Ruberti, Dynamic shear-influenced collagen self-assembly. Biomaterials 30(34), 6581–6592 (2009)
U. Schaff, M. Xing, K. Lin, N. Pan, N. Jeon, S. Simon, Vascular mimetics based on microfluidics for imaging the leukocyte— endothelial inflammatory response. Lab Chip 7(4), 448–456 (2007)
B. Schmidt, P. Huang, K. Breuer, M. Lawrence, Catch strip assay for the relative assessment of two-dimensional protein association kinetics. Anal Chem 80(4), 944–950 (2008)
H. Slayter, J. Loscalzo, P. Bockenstedt, R.I. Handin, Native Conformation of Human Vonwillebrand Protein— Analysis by Electron-Microscopy and Quasi-Elastic Light-Scattering. J Biol Chem 260, 8559–8563 (1985)
S.H. Tam, P.M. Sassoli, R.E. Jordan, M.T. Nakada, Abciximab (ReoPro, chimeric 7E3 Fab) demonstrates equivalent affinity and functional blockade of glycoprotein IIb/IIIa and alpha(v)beta(3) integrins. Circulation 98, 1085–1091 (1998)
P. Tengvall, I. Lundstrom, B. Liedberg, Protein adsorption studies on model organic surfaces: an ellipsometric and infrared spectroscopic approach. Biomaterials 19, 407–422 (1998a)
P. Tengvall, A. Askendal, I. Lundstrom, Studies on protein adsorption and activation of complement on hydrated aluminium surfaces in vitro. Biomaterials 19, 935–940 (1998b)
W. Thomas, Catch bonds in adhesion. Annu Rev Biomed Eng 10, 39–57 (2008)
A. Toscano, M.M. Santore, Fibrinogen adsorption on three silica-based surfaces: Conformation and kinetics. Langmuir 22, 2588–2597 (2006)
G.M. Whitesides, E. Ostuni, S. Takayama, X.Y. Jiang, D.E. Ingber, Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3, 335–373 (2001)
P. Yager, G.J. Domingo, J. Gerdes, Point-of-care diagnostics for global health. Annu Rev Biomed Eng 10, 107–144 (2008)
J.J. Zwaginga, G. Nash, M.R. King, J.W. Heemskerk, M. Frojmovic, M.F. Hoylaerts, K.S. Sakariassen, Flow-based assays for global assessment of hemostasis. Part 1: Biorheologic considerations. J Thromb Haemost 4, 2486–7 (2006a)
J.J. Zwaginga, K.S. Sakariassen, G. Nash, M.R. King, J.W. Heemskerk, M. Frojmovic, M.F. Hoylaerts, Flow-based assays for global assessment of hemostasis. Part 2: current methods and considerations for the future. J Thromb Haemost 4, 2716–7 (2006b)
J.J. Zwaginga, K.S. Sakarjassen, M.R. King, T.G. Diacovo, E.F. Grabowski, G. Nash, M. Hoylaerts, J.W. Heemskerk, Can blood flow assays help to identify clinically relevant differences in von Willebrand factor functionality in von Willebrand disease types 1–3? J Thromb Haemost 5, 2547–2549 (2007)
Acknowledgements
The authors gratefully acknowledge the help of Dr. Cedric Volcke with the AFM measurements, and related discussions, and the help of Dr. Helen Berney and Dr. Arun Harish, Field Applications Scientist, Farfield Group Ltd., UK with the dual-polarized interferometry and related discussions. This material is based upon works supported by the Science Foundation Ireland under Grant No. 05/CE3/B754.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary materials
Rights and permissions
About this article
Cite this article
Kent, N.J., Basabe-Desmonts, L., Meade, G. et al. Microfluidic device to study arterial shear-mediated platelet-surface interactions in whole blood: reduced sample volumes and well-characterised protein surfaces. Biomed Microdevices 12, 987–1000 (2010). https://doi.org/10.1007/s10544-010-9453-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-010-9453-y