Abstract
Purpose
Shear-mediated thrombosis is a clinically relevant phenomenon that underlies excessive arterial thrombosis and device-induced thrombosis. Red blood cells are known to mechanically contribute to physiological hemostasis through margination of platelets and vWF, facilitating the unfurling of vWF multimers, and increasing the fraction of thrombus-contacting platelets. Shear also plays a role in this phenomenon, increasing both the degree of margination and the near-wall forces experienced by vWF and platelets leading to unfurling and activation. Despite this, the contribution of red blood cells in shear-induced platelet aggregation has not been fully investigated—specifically the effect of elevated hematocrit has not yet been demonstrated.
Methods
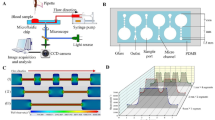
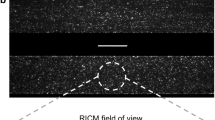
Here, a microfluidic model of a sudden expansion is presented as a platform for investigating platelet adhesion at hematocrits ranging from 0 to 60% and shear rates ranging from 1000 to 10,000 s−1. The sudden expansion geometry models nonphysiological flow separation characteristic to mechanical circulatory support devices, and the validatory framework of the FDA benchmark nozzle. PDMS microchannels were fabricated and coated with human collagen. Platelets were fluorescently tagged, and blood was reconstituted at variable hematocrit prior to perfusion experiments. Integrin function of selected blood samples was inhibited by a blocking antibody, and platelet adhesion and aggregation over the course of perfusion was monitored.
Results
Increasing shear rates at physiological and elevated hematocrit levels facilitate robust platelet adhesion and formation of large aggregates. Shear-induced platelet aggregation is demonstrated to be dependent on both αIIbβIII function and the presence of red blood cells. Inhibition of αIIbβIII results in an 86.4% reduction in overall platelet adhesion and an 85.7% reduction in thrombus size at 20-60% hematocrit. Hematocrit levels of 20% are inadequate for effective platelet margination and subsequent vWF tethering, resulting in notable decreases in platelet adhesion at 5000 and 10,000 s-1 compared to 40% and 60%. Inhibition of αIIbβIII triggered dramatic reductions in overall thrombus coverage and large aggregate formation. Stability of platelets tethered by vWF are demonstrated to be αIIbβIII-dependent, as adhesion of single platelets treated with A2A9, an anti-αIIbβIII blocking antibody, is transient and did not lead to sustained thrombus formation.
Conclusions
This study highlights driving factors in vWF-mediated platelet adhesion that are relevant to clinical suppression of shear-induced thrombosis and in vitro assays of platelet adhesion. Primarily, increasing hematocrit promotes platelet margination, permitting shear-induced platelet aggregation through αIIbβIII-mediated adhesion at supraphysiological shear rates.








Similar content being viewed by others
Data availability
Data will be made available by the corresponding author (K.B. Manning) upon reasonable request.
References
Sadler, J. E. Biochemistry and genetics of von Willebrand factor, 1998.
Siedlecki, C. A., B. J. Lestini, K. K. Kottke-Marchant, S. J. Eppell, D. L. Wilson, and R. E. Marchant. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 88:2939–2950, 1996.
Ruggeri, Z. M., and J. Ware. von Willebrand factor. FASEB J. 7:308–316, 1993. https://doi.org/10.1096/fasebj.7.2.8440408.
Fu, H., Y. Jiang, D. Yang, F. Scheiflinger, W. P. Wong, and T. A. Springer. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat Commun. 2017. https://doi.org/10.1038/s41467-017-00230-2.
Pareti, F. I., Y. Fujimura, J. A. Dent, L. Z. Holland, T. S. Zimmerman, and Z. M. Ruggeri. Isolation and characterization of a collagen binding domain in human von Willebrand factor. J Biol Chem. 261:15310–15315, 1986. https://doi.org/10.1016/S0021-9258(18)66869-3.
Houdijk, W. P. M., K. S. Sakariassen, P. F. E. M. Nievelstein, and J. J. Sixma. Role of Factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J Clin Investig. 75:531–540, 1985. https://doi.org/10.1172/JCI111729.
Liu, Z. L., D. N. Ku, and C. K. Aidun. Mechanobiology of shear-induced platelet aggregation leading to occlusive arterial thrombosis: a multiscale in silico analysis. J Biomech. 2021. https://doi.org/10.1016/j.jbiomech.2021.110349.
Casa, L. D. C., D. N. Ku, and G. W. Woodruff. Thrombus formation at high shear rates. Annu Rev Biomed Eng. 2017. https://doi.org/10.1146/annurev-bioeng-071516.
Westein, E., A. D. Van Der Meer, M. J. E. Kuijpers, J. P. Frimat, A. Van Den Berg, and J. W. M. Heemskerk. Atherosclerotic geometries exacerbate pathological thrombus formation poststenosis in a von Willebrand factor-dependent manner. Proc Natl Acad Sci USA. 110:1357–1362, 2013. https://doi.org/10.1073/pnas.1209905110.
Manning, K. B., F. Nicoud, and S. M. Shea. Mathematical and computational modeling of device-induced thrombosis. Curr Opin Biomed Eng. 20:100349, 2021. https://doi.org/10.1016/J.COBME.2021.100349.
Kreuziger, L. B., M. S. Slaughter, K. Sundareswaran, and A. E. Mast. Clinical relevance of histopathologic analysis of heart mate II thrombi. ASAIO J. 64:754–759, 2018. https://doi.org/10.1097/MAT.0000000000000759.
Consolo, F., A. Dimasi, M. Rasponi, L. Valerio, F. Pappalardo, D. Bluestein, M. J. Slepian, G. B. Fiore, and A. Redaelli. Microfluidic approaches for the assessment of blood cell trauma: A focus on thrombotic risk in mechanical circulatory support devices. Int J Artif Organs. 39:184–193, 2016. https://doi.org/10.5301/ijao.5000485.
Kim, D. A., and D. N. Ku. Structure of shear-induced platelet aggregated clot formed in an in vitro arterial thrombosis model. Blood Adv. 6:2872–2883, 2022. https://doi.org/10.1182/bloodadvances.2021006248.
Colace, T. V., and S. L. Diamond. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 33:105–113, 2013. https://doi.org/10.1161/ATVBAHA.112.300522.
Scavone, M., S. Bozzi, T. Mencarini, G. Podda, M. Cattaneo, and A. Redaelli. Platelet adhesion and thrombus formation in microchannels: the effect of assay-dependent variables. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21030750.
Receveur, N., D. Nechipurenko, Y. Knapp, A. Yakusheva, E. Maurer, C. V. Denis, F. Lanza, M. Panteleev, C. Gachet, and P. H. Mangin. Shear rate gradients promote a bi-phasic thrombus formation on weak adhesive proteins, such as fibrinogen in a von Willebrand factor-dependent manner. Haematologica. 105:2471–2483, 2020. https://doi.org/10.3324/haematol.2019.235754.
Rack, K., V. Huck, M. Hoore, D. A. Fedosov, S. W. Schneider, and G. Gompper. Margination and stretching of von Willebrand factor in the blood stream enable adhesion. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-14346-4.
Zhao, R., M. V. Kameneva, and J. F. Antaki. Investigation of platelet margination phenomena at elevated shear stress. Biorheology. 44:161–177, 2007.
Walton, B. L., M. Lehmann, T. Skorczewski, L. A. Holle, J. D. Beckman, J. A. Cribb, M. J. Mooberry, A. R. Wufsus, B. C. Cooley, J. W. Homeister, R. Pawlinski, M. R. Falvo, N. S. Key, A. L. Fogelson, K. B. Neeves, and A. S. Wolberg. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 129:2537–2546, 2017. https://doi.org/10.1182/blood-2016-10-746479.
Tilles, A. W., and E. C. Eckstein. The near-wall excess of platelet-sized particles in blood flow: its dependence on hematocrit and wall shear rate. Microvasc Res. 33:211–223, 1987. https://doi.org/10.1016/0026-2862(87)90018-5.
Lehmann, M., R. M. Schoeman, P. J. Krohl, A. M. Wallbank, J. R. Samaniuk, M. Jandrot-Perrus, and K. B. Neeves. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI–dependent manner in an in vitro venous thrombosis model. Arterioscler Thromb Vasc Biol. 38:1052–1062, 2018. https://doi.org/10.1161/ATVBAHA.118.310731.
Qi, Q. M., E. Dunne, I. Oglesby, I. Schoen, A. J. Ricco, D. Kenny, and E. S. G. Shaqfeh. In vitro measurement and modeling of platelet adhesion on VWF-coated surfaces in channel flow. Biophys J. 116:1136–1151, 2019. https://doi.org/10.1016/j.bpj.2019.01.040.
Wang, Y., M. Morabito, X. F. Zhang, E. Webb, A. Oztekin, and X. Cheng. Shear-induced extensional response behaviors of tethered von Willebrand factor. Biophys J. 116:2092–2102, 2019. https://doi.org/10.1016/j.bpj.2019.04.025.
Zhussupbekov, M., R. Méndez Rojano, W.-T. Wu, and J. F. Antaki. von Willebrand factor unfolding mediates platelet deposition in a model of high-shear thrombosis. Biophys J. 121:4033–4047, 2022. https://doi.org/10.1016/j.bpj.2022.09.040.
Sing, C. E., and A. Alexander-Katz. Elongational flow induces the unfolding of von willebrand factor at physiological flow rates. Biophys J. 2010. https://doi.org/10.1016/j.bpj.2010.01.032.
Goodman, P. D., E. T. Barlow, P. M. Crapo, S. F. Mohammad, and K. A. Solen. Computational model of device-induced thrombosis and thromboembolism. Ann Biomed Eng. 33:780–797, 2005. https://doi.org/10.1007/s10439-005-2951-z.
Yang, L., T. Neuberger, and K. B. Manning. In vitro real-time magnetic resonance imaging for quantification of thrombosis. Magn Reson Mater Phys Biol Med. 34:285–295, 2021. https://doi.org/10.1007/s10334-020-00872-2.
Taylor, J. O., R. S. Meyer, S. Deutsch, Keefe, and B. Manning. Development of a computational model for macroscopic predictions of device-induced thrombosis A. Biomech Model Mechanobiol. 15:1713–1731, 2016. https://doi.org/10.1007/s10237-016-0793-2.
Yang, L., N. Tobin, and K. B. Manning. Refining a numerical model for device-induced thrombosis and investigating the effects of non-Newtonian blood models. J Biomech. 120:110393, 2021. https://doi.org/10.1016/j.jbiomech.2021.110393.
Jamiolkowski, M. A., J. R. Woolley, M. V. Kameneva, J. F. Antaki, and W. R. Wagner. Real time visualization and characterization of platelet deposition under flow onto clinically relevant opaque surfaces. J Biomed Mater Res A. 103:1303–1311, 2015. https://doi.org/10.1002/jbm.a.35202.
Dimasi, A., M. Rasponi, J. Sheriff, W. C. Chiu, D. Bluestein, P. L. Tran, M. J. Slepian, and A. Redaelli. Microfluidic emulation of mechanical circulatory support device shear-mediated platelet activation. Biomed Microdevices. 17:1–11, 2015. https://doi.org/10.1007/s10544-015-0015-1.
Jamiolkowski, M. A., D. D. Pedersen, W.-T.T. Wu, J. F. Antaki, and W. R. Wagner. Visualization and analysis of biomaterial-centered thrombus formation within a defined crevice under flow. Biomaterials. 96:72–83, 2016. https://doi.org/10.1016/j.biomaterials.2016.04.022.
Brækkan, S. K., E. B. Mathiesen, I. Njølstad, T. Wilsgaard, and J. B. Hansen. Hematocrit and risk of venous thromboembolism in a general population. The Tromsø study. Haematologica. 95:270–275, 2010. https://doi.org/10.3324/haematol.2009.008417.
Fitzgibbon, S., A. P. Spann, Q. M. Qi, and E. S. G. Shaqfeh. In vitro measurement of particle margination in the microchannel flow: effect of varying hematocrit. Biophys J. 108:2601–2608, 2015. https://doi.org/10.1016/j.bpj.2015.04.013.
Liu, Z. L., C. Bresette, C. K. Aidun, and D. N. Ku. SIPA in 10 milliseconds: VWF tentacles agglomerate and capture platelets under high shear. Blood Adv. 6:2453–2465, 2022. https://doi.org/10.1182/bloodadvances.2021005692.
Ldc, C., and G. Se. Relative contributions of von Willebrand factor and platelets in high shear thrombosis. J Hematol Thromb Dis. 2016. https://doi.org/10.4172/2329-8790.1000249.
Kushchenko, Y. K., and A. V. Belyaev. Effects of hydrophobicity, tethering and size on flow-induced activation of von Willebrand factor multimers. J Theor Biol. 2020. https://doi.org/10.1016/j.jtbi.2019.110050.
Belyaev, A. V., and Y. K. Kushchenko. Biomechanical activation of blood platelets via adhesion to von Willebrand factor studied with mesoscopic simulations. Biomech Model Mechanobiol. 2023. https://doi.org/10.1007/s10237-022-01681-3.
Belyaev, A. V: Computer modelling of initial platelet adhesion during microvascular thrombosis. Russ J Numer Anal Math Model. 2019. https://doi.org/10.1515/rnam-2019-0020.
Bennett, J. S., J. A. Hoxie, S. F. Leitman, G. Vilaire, and D. B. Cines. Inhibition of fibrinogen binding to stimulated human platelets by monoclonal antibody. Proc Natl Acad Sci USA. 80:2417–2421, 1983. https://doi.org/10.1073/pnas.80.9.2417.
Bennett, J. S., S. J. Shattil, J. W. Power, and T. K. Gartner. Interaction of fibrinogen with its platelet receptor. Differential effects of α and γ chain fibrinogen peptides on the glycoprotein IIb-IIIa complex. J Biol Chem. 263:12948–12953, 1988. https://doi.org/10.1016/s0021-9258(18)37654-3.
HEPES-buffered saline. Cold Spring Harb Protoc. 2006, pdb.rec8786 (2006). https://doi.org/10.1101/pdb.rec8786
Milionis, A. S. I., Fragouli, D., Brandi, F., Athanassiou, A. Combination of lithography and coating methods for surface wetting control. In: Updates in Advanced Lithography. InTech (2013)
Hansen, R. R., A. A. Tipnis, T. C. White-Adams, J. A. Di Paola, and K. B. Neeves. Characterization of collagen thin films for von Willebrand factor binding and platelet adhesion. Langmuir. 27:13648–13658, 2011. https://doi.org/10.1021/la2023727.
Sakariassen, K. S., L. Orning, and V. T. Turitto. The impact of blood shear rate on arterial thrombus formation. Future Sci OA. 2015. https://doi.org/10.4155/fso.15.28.
Neeves, K. B., S. F. Maloney, K. P. Fong, A. A. Schmaier, M. L. Kahn, L. F. Brass, and S. L. Diamond. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. Journal of Thrombosis and Haemostasis. 6:2193–2201, 2008. https://doi.org/10.1111/j.1538-7836.2008.03188.x.
Restle, D. J., D. M. Zhang, G. Hung, J. L. Howard, F. Kallel, M. A. Acker, P. Atluri, and C. R. Bartoli. Preclinical models for translational investigations of left ventricular assist device-associated von Willebrand factor degradation. Artif Organs. 39:569–575, 2015. https://doi.org/10.1111/aor.12428.
Hariharan, P., M. Giarra, V. Reddy, S. W. Day, K. B. Manning, S. Deutsch, S. F. C. Stewart, M. R. Myers, M. R. Berman, G. W. Burgreen, E. G. Paterson, and R. A. Malinauskas. Multilaboratory particle image velocimetry analysis of the FDA benchmark nozzle model to support validation of computational fluid dynamics simulations. J Biomech Eng. 2011. https://doi.org/10.1115/1.4003440.
Spann, A. P., J. E. Campbell, S. R. Fitzgibbon, A. Rodriguez, A. P. Cap, L. H. Blackbourne, and E. S. G. Shaqfeh. The effect of hematocrit on platelet adhesion: experiments and simulations. Biophys J. 111:577–588, 2016. https://doi.org/10.1016/j.bpj.2016.06.024.
Chen, H., J. I. Angerer, M. Napoleone, A. J. Reininger, S. W. Schneider, A. Wixforth, M. F. Schneider, and A. Alexander-Katz. Hematocrit and flow rate regulate the adhesion of platelets to von Willebrand factor. Biomicrofluidics. 2013. https://doi.org/10.1063/1.4833975.
Vignoli, A., S. Gamba, P. E. J. van der Meijden, M. Marchetti, L. Russo, S. Tessarolo, C. Giaccherini, F. Swieringa, H. Ten Cate, G. Finazzi, J. W. M. Heemskerk, and A. Falanga. Increased platelet thrombus formation under flow conditions in whole blood from polycythaemia vera patients. Blood Transfus. 20:143–151, 2022. https://doi.org/10.2450/2021.0456-20.
Woolley, J. R., J. J. Teuteberg, C. A. Bermudez, J. K. Bhama, K. L. Lockard, R. L. Kormos, and W. R. Wagner. Temporal leukocyte numbers and granulocyte activation in pulsatile and rotary ventricular assist device patients. Artif Organs. 38:447–455, 2014. https://doi.org/10.1111/aor.12200.
Long, J. A., A. Ündar, K. B. Manning, and S. Deutsch. Viscoelasticity of pediatric blood and its implications for the testing of a pulsatile pediatric blood pump. ASAIO J. 51:563–566, 2005. https://doi.org/10.1097/01.mat.0000180353.12963.f2.
Denis, C., J. A. Williams, X. Lu, D. Meyer, and D. Baruch. Solid-phase von Willebrand factor contains a conformationally active RGD motif that mediates endothelial cell adhesion through the α(v)β3 receptor. Blood. 82:3622–3630, 1993. https://doi.org/10.1182/blood.v82.12.3622.3622.
Watt, K. W. K., T. Takagi, R. F. Doolittle, and T. Takagi. Amino acid sequence of the β chain of human fibrinogen. Biochemistry. 18:68–76, 1979. https://doi.org/10.1021/bi00568a011.
Zhao, R., J. N. Marhefka, F. Shu, S. J. Hund, M. V. Kameneva, and J. F. Antaki. Micro-flow visualization of red blood cell-enhanced platelet concentration at sudden expansion. Ann Biomed Eng. 36:1130–1141, 2008. https://doi.org/10.1007/s10439-008-9494-z.
Schneider, S. W., Nuschele, S., Wixforth, A., Gorzelanny, C., Alexander-Katz, A., Netz, R. R., Schneider, M. F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers (2007)
Savage, B., Sixma, J. J., Ruggeri, Z. M.: Functional self-association of von Willebrand factor during platelet adhesion under flow (2002)
Nesbitt, W. S., E. Westein, F. J. Tovar-Lopez, E. Tolouei, A. Mitchell, J. Fu, J. Carberry, A. Fouras, and S. P. Jackson. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 15:665–673, 2009. https://doi.org/10.1038/nm.1955.
Rana, A., Westein, E., Niego, B., Hagemeyer, C. E. Shear-dependent platelet aggregation: mechanisms and therapeutic opportunities, 2019.
Fu, H., Jiang, Y., Wong, W. P., Springer, T. A. Single-molecule imaging of von Willebrand factor reveals tension-dependent self-association, 2021.
Dütting, S., M. Bender, and B. Nieswandt. Platelet GPVI: a target for antithrombotic therapy?! Trends Pharmacol Sci. 33:583–590, 2012. https://doi.org/10.1016/J.TIPS.2012.07.004.
Lin, J., Sorrells, M. G., Lam, W. A., Neeves, K. B. Physical forces regulating hemostasis and thrombosis: vessels, cells, and molecules in illustrated review, 2021.
Bozzi, S., Y. Roka-Moiia, T. Mencarini, F. Vercellino, I. Epifani, K. R. Ammann, F. Consolo, M. J. Slepian, and A. Redaelli. Characterization of the competing role of surface-contact and shear stress on platelet activation in the setting of blood contacting devices. Int J Artif Organs. 44:1013–1020, 2021. https://doi.org/10.1177/03913988211009909.
Ding, J., Z. Chen, S. Niu, J. Zhang, N. K. Mondal, B. P. Griffith, and Z. J. Wu. Quantification of shear-induced platelet activation: high shear stresses for short exposure time. Artif Organs. 39:576–583, 2015. https://doi.org/10.1111/aor.12438.
Reimers, R. C., S. P. Sutera, and J. H. Joist. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood. 64:1200–1206, 1984.
Goldsmith, H., E. Kaufer, and F. McIntosh. Effect of hematocrit on adenosine diphosphate-induced aggregation of human platelets in tube flow. Biorheology. 32:537–552, 1995. https://doi.org/10.1016/0006-355X(95)00031-4.
Goldsmith, H. L., D. N. Bell, S. Braovac, A. Steinberg, and F. McIntosh. Physical and chemical effects of red cells in the shear-induced aggregation of human platelets. Biophys J. 69:1584–1595, 1995. https://doi.org/10.1016/S0006-3495(95)80031-7.
Acknowledgments
This work is supported, in part, by NIH HL136369.The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank Jessica C. Cardenas (University of Texas Health Science Center at Houston) for generously reading this manuscript.
Funding
This work is supported, in part, by NIH HL136369. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR002014.
Author information
Authors and Affiliations
Contributions
K.B.M. and A.R. conceived and designed study; C.T.W., S.C.W., and S.A.R. performed experimental studies; C.T.W. analyzed data, interpreted experimental results, generated figures, and drafted manuscript; C.T.W., K.B.M. and A.R. edited and revised manuscript; K.B.M applied for and obtained funding; C.T.W., S.C.W., S.A.R., A.R., and K.B.M. approved final manuscript.
Corresponding author
Ethics declarations
Competing interest
K.B.M. has a financial interest in Cranial Devices, Inc, a company which could potentially benefit from the results of this research. The interest has been reviewed and is being managed by the University in accordance with its individual Conflict of Interest policy, for the purpose of maintaining the objectivity of research at The Pennsylvania State University. All other authors declare no conflicts.
Informed Consent
Informed consent was obtained from all human subjects in a Pennsylvania State University IRB approved protocol.
Additional information
Associate Editor Scott Simon oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watson, C.T., Ward, S.C., Rizzo, S.A. et al. Influence of Hematocrit Level and Integrin αIIbβIII Function on vWF-Mediated Platelet Adhesion and Shear-Induced Platelet Aggregation in a Sudden Expansion. Cel. Mol. Bioeng. 17, 49–65 (2024). https://doi.org/10.1007/s12195-024-00796-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-024-00796-0