Abstract
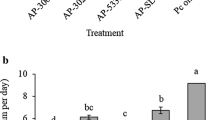
The effect of inoculation of strawberry roots by two entomopathogenic fungal isolates, Metarhizium robertsii (ESALQ 1622) and Beauveria bassiana (ESALQ 3375), on naturally occurring arthropod pests and plant diseases was investigated in four commercial strawberry fields during two growing seasons in Brazil. Three locations represented open-field production while strawberries were grown in low tunnels at the fourth location. Population responses of predatory mites to the fungal treatments were also assessed. Plants inoculated by the fungal isolates resulted in significantly fewer Tetranychus urticae adults compared to control plants at all four locations. The mean cumulative numbers ± SE of T. urticae per leaflet were: M. robertsii (225.6 ± 59.32), B. bassiana (206.5 ± 51.48) and control (534.1 ± 115.55) at the three open-field locations, while at the location with tunnels numbers were: M. robertsii (79.7 ± 10.02), B. bassiana (107.7 ± 26.85) and control (207.4 ± 49.90). Plants treated with B. bassiana had 50% fewer leaves damaged by Coleoptera, while there were no effects on numbers of whiteflies and thrips. Further, lower proportions of leaflets with symptoms of the foliar plant pathogenic fungi Mycosphaerella fragariae and Pestalotia longisetula were observed in the M. robertsii (4.6% and 1.3%)- and B. bassiana (6.1% and 1.3%)-treated plots compared to control plots (9.8% and 3.7%). No effect was seen on numbers of naturally occurring predatory mites. Our results suggest that both isolates tested may be used as root inoculants of strawberries to protect against foliar pests, particularly spider mites, and also against foliar plant pathogenic fungi without harming naturally occurring and beneficial predatory mites.



Similar content being viewed by others
References
Akello J, Sikora R (2012) Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol Control 61:215–221. https://doi.org/10.1016/j.biocontrol.2012.02.007
Akutse KS, Maniania NK, Fiaboe KKM, Van Den Berg J, Ekesi S (2013) Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol 6:293–301. https://doi.org/10.1016/j.funeco.2013.01.003
Akutse KS, Fiaboe KKM, Van den Berg J, Ekesi S, Maniania NK (2014) Effects of endophyte colonization of Vicia faba (Fabaceae) plants on the life-history of leafminer parasitoids Phaedrotoma scabriventris (Hymenoptera: Braconidae) and Diglyphus isaea (Hymenoptera: Eulophidae). PLoS ONE 9:e109965. https://doi.org/10.1371/journal.pone.0109965
Andreazza F, Haddi K, Oliveira EE, Ferreira JAM (2016) Drosophila suzukii (Diptera: Drosophilidae) arrives at Minas Gerais State, a main strawberry production region in Brazil. Fla Entomol 99:796–798. https://doi.org/10.1653/024.099.0439
Ansari MA, Butt TM (2013) Influence of the application methods and doses on the susceptibility of black vine weevil larvae Otiorhynchus sulcatus to Metarhizium anisopliae in field-grown strawberries. Biocontrol 58:257–267. https://doi.org/10.1007/s10526-012-9491-x
Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC (2013) A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J Pest Sci 86:361–386. https://doi.org/10.1007/s10340-013-0503-0
Barzman M, Bàrberi P, Birch ANE, Boonekamp P, Dachbrodt-Saaydeh S, Graf B, Hommel B, Jensen JE, Kiss J, Kudsk P, Lamichhane JR, Messéan A, Moonen AC, Ratnadass A, Ricci P, Sarah JL, Sattin M (2015) Eight principles of integrated pest management. Agron Sustain Dev 35:1199–1215. https://doi.org/10.1007/s13593-015-0327-9
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–28. https://doi.org/10.18637/jss.v067.i01
Behie SW, Jones SJ, Bidochka MJ (2015) Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol 13:112–119. https://doi.org/10.1016/j.funeco.2014.08.001
Bernardi D, Botton M, Nava DE, Zawadneak MAC (2015) Guia para a identificação e monitoramento de pragas e seus inimigos naturais em morangueiro. Embrapa Clima Temperado, Brasília
Bing LA, Lewis LC (1991) Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol 20:1207–1211. https://doi.org/10.1093/ee/20.4.1207
Bixby-Brosi AJ, Potter DA (2012) Endophyte-mediated tritrophic interactions between a grass-feeding caterpillar and two parasitoid species with different life histories. Arthropod Plant Interact 6:27–34. https://doi.org/10.1007/s11829-011-9163-2
Brotman YL, Landau U, Cuadros-Inostroza A, Takayuki T, Fernie AR, Chet I, Viterbo A, Willmitzer L (2013) Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog 9:e1003221. https://doi.org/10.1371/journal.ppat.1003221
Canassa F, Tall S, Moral RA, Lara IAR, Delalibera I Jr, Meyling NV (2019) Effects of bean seed treatment by the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana on plant growth, spider mite populations and behavior of predatory mites. Biol Control 132:199–208. https://doi.org/10.1016/j.biocontrol.2019.02.003
Carroll G (1988) Fungal endophytes in stems and leaves—from latent pathogen to mutualistic symbiont. Ecology 69:2–9. https://doi.org/10.2307/1943154
Castillo-Lopez D, Zhu-Salzman K, Ek-Ramos MJ, Sword GA (2014) The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 9:e103891. https://doi.org/10.1371/journal.pone.0103891
Castro TR, Wekesa VW, Moral RA, Demétrio CGB, Delalibera I Jr, Klingen I (2013) The effects of photoperiod and light intensity on the sporulation of Brazilian and Norwegian isolates of Neozygites floridana. J Invertebr Pathol 114:230–233. https://doi.org/10.1016/j.jip.2013.08.004
Castro T, Mayerhofer J, Enkerlib J, Eilenberg J, Meyling NV, Moral RA, Demétrio CGB, Delalibera I Jr (2016) Persistence of Brazilian isolates of the entomopathogenic fungi Metarhizium anisopliae and M. robertsii in strawberry crop soil after soil drench application. Agric Ecosyst Environ 233:361–369. https://doi.org/10.1016/j.agee.2016.09.031
Castro T, Eilenberg J, Delalibera I Jr (2018) Exploring virulence of new and less studied species of Metarhizium spp. from Brazil for two-spotted spider mite control. Exp Appl Acarol 74:139–146. https://doi.org/10.1007/s10493-018-0222-6
Chant DA, Yoshida-Shaul E (1991) Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int J Acarol 17(3):187–199. https://doi.org/10.1080/01647959108683906
Coll M, Shakya S, Shouster I, Nenner Y, Steinberg S (2007) Decision-making tools for Frankliniella occidentalis management in strawberry: consideration of target markets. Entomol Exp Appl 122:59–67. https://doi.org/10.1111/j.1570-7458.2006.00488.x
Czaja K, Góralczyk K, Struciński P, Hernik A, Korcz W, Minorczyk M, Łyczewska M, Ludwicki JK (2015) Biopesticides—towards increased consumer safety in the European Union. Pest Manag Sci 71:3–6. https://doi.org/10.1002/ps.3829
Dara SK (2016) Managing strawberry pests with chemical pesticides and non-chemical alternatives. Int J Fruit Sci 16:1–13. https://doi.org/10.1080/15538362.2016.1195311
Demétrio CGB, Hinde J, Moral RA (2014) Models for overdispersed data in entomology. In: Ferreira CP, Godoy WAC (eds) Ecological modelling applied to entomology, 1st edn. Springer, New York, pp 219–259
Easterbrook MA, Fitzgerald JD, Solomon MG (2001) Biological control of strawberry tarsonemid mite Phytonemus pallidus and two-spotted spider mite Tetranychus urticae on strawberry in the UK using species of Neoseiulus (Amblyseius) (Acari: Phytoseiidae). Exp Appl Acarol 25:25–36. https://doi.org/10.1023/A:1010685903130
Eilenberg J, Hajek A, Lomer C (2001) Suggestions for unifying the terminology in biological control. Biocontrol 46:387–400. https://doi.org/10.1023/A:1014193329979
FAOSTAT (2018) Food and Agriculture Organization of the United Nations Statistics. http://faostat.org/. Accessed 10 Oct 2018
Fatoretto MB, Moral RA, Demétrio CGB, de Pádua CS, Menarin V, Rojas VMA, D’Alessandro CP, Delalibera I Jr (2018) Overdispersed fungus germination data: statistical analysis using R. Biocontrol Sci Technol 28:1034–1053. https://doi.org/10.1080/09583157.2018.1504888
Garcia R, Caltagirone LE, Gutierrez AP (1988) Comments on a redefinition of biological control. Bioscience 38:692–694. https://doi.org/10.2307/1310871
Garrido C, Carbú M, Fernández-Acero FJ, González-Rodríguez VE, Cantoral JM (2011) New insights in the study of strawberry fungal pathogens. In: Husaini AM, Mercado JA (eds) Genes, genomes and genomics, 1st edn. Global Science Books, Takamatsu, pp 24–39
Garrido-Jurado I, Resquín-Romero G, Amarilla SP, Ríos-Moreno A, Carrasco L, Quesada-Moraga E (2017) Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J Pest Sci 90:319–330. https://doi.org/10.1007/s10340-016-0767-2
Gathage JW, Lagat ZO, Fiaboe KKM, Akutse KS, Ekesi S, Maniania NK (2016) Prospects of fungal endophytes in the control of Liriomyza leafminer flies in common bean Phaseolus vulgaris under field conditions. Biocontrol 61:741–753. https://doi.org/10.1007/s10526-016-9761-0
Goettel MS, Poprawski TJ, Vandenverg JD, Li Z, Roberts DW (1990) Safety to nontarget invertebrates of fungal biocontrol agents. In: Laird M, Lacey LA, Davison EW (eds) Safety of microbial insecticides, 1st edn. CRC Press, Flórida, pp 209–232
González-Mas N, Cuenca-Medina M, Gutierrez-Sanchez F, Quesada-Moraga E (2019) Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J Pest Sci 92:1271–1281. https://doi.org/10.1007/s10340-019-01098-5
Greco NM, Pereyra PC, Guillade A (2005) Host-plant acceptance and performance of Tetranychus urticae (Acari: Tetranychidae). J Appl Entomol 130:32–36. https://doi.org/10.1111/j.1439-0418.2005.01018.x
Greenfield M, Gomez-Jimenez MI, Ortiz V, Vega FE, Kramer M, Parsa S (2016) Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol Control 95:40–48. https://doi.org/10.1016/j.biocontrol.2016.01.002
Hajek AE, Delalibera I Jr (2010) Fungal pathogens as classical biological control agents against arthropods. Biocontrol 55:147–158. https://doi.org/10.1007/s10526-009-9253-6
Hoffmann D, Vierheilig H, Schausberger P (2011) Arbuscular mycorrhiza enhances preference of ovipositing predatory mites for direct prey-related cues. Physiol Entomol 36:90–95. https://doi.org/10.1111/j.1365-3032.2010.00751.x
Jaber LR, Alananbeh KM (2018) Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol Control 126:117–126. https://doi.org/10.1016/j.biocontrol.2018.08.007
Jaber LR, Araj SE (2018) Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol Control 116:53–61. https://doi.org/10.1016/j.biocontrol.2017.04.005
Jaber LR, Ownley BH (2018) Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol Control 116:36–45. https://doi.org/10.1016/j.biocontrol.2017.01.018
Keyser CA, Jensen B, Meyling NV (2016) Dual effects of Metarhizium spp. and Clonostachys rosea against an insect and a seed-borne pathogen in wheat. Pest Manag Sci 72:517–526. https://doi.org/10.1002/ps.4015
Klingen I, Haukeland S (2006) The soil as a reservoir for natural enemies of pest insects and mites with emphasis on fungi and nematodes. In: Eilenberg J, Hokkanen HMT (eds) An ecological and societal approach to biological control, 1st edn. Springer, Dordrecht, pp 145–211
Klingen I, Westrum K (2007) The effect of pesticides used in strawberries on the phytophagous mite Tetranychus urticae (Acari: Tetranychidae) and its fungal natural enemy Neozygites floridana (Zygomycetes: Entomophthorales). Biol Control 43:222–230. https://doi.org/10.1016/j.biocontrol.2007.07.013
Klingen I, Westrum K, Meyling N (2015) Effect of Norwegian entomopathogenic fungal isolates against Otiorhynchus sulcatus larvae at low temperatures and persistence in strawberry rhizospheres. Biol Control 81:1–7. https://doi.org/10.1016/j.biocontrol.2014.10.006
Kuhn TMA, Loeck AE, Zawadneak MAC, Garcia MS, Botton M (2014) Parâmetros biológicos e tabela de vida de fertilidade de Neopamera bilobata (Hemiptera: Rhyparochromidae) em morangueiro. Pesq Agropec Bras 49:422–427. https://doi.org/10.1590/S0100-204X2014000600003
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41. https://doi.org/10.1016/j.jip.2015.07.009
Mantzoukas S, Chondrogiannis C, Grammatikopoulos G (2015) Effects of three endophytic entomopathogens on sweet sorghum and on the larvae of the stalk borer Sesamia nonagrioides. Entomol Exp Appl 154:78–87. https://doi.org/10.1111/eea.12262
McKinnon AC, Saari S, Moran-Diez ME, Meyling NV, Raad M, Glare TR (2017) Beauveria bassiana as an endophyte: a critical review on associated methodology and biocontrol potential. Biocontrol 62:1–17. https://doi.org/10.1007/s10526-016-9769-5
Meyling NV, Eilenberg J (2007) Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol Control 43:145–155. https://doi.org/10.1016/j.biocontrol.2007.07.007
Meyling N, Hajek A (2010) Principles from community and metapopulation ecology: application to fungal entomopathogens. Biol Control 55:39–54. https://doi.org/10.1007/s10526-009-9246-5
Moraes GJ, McMurtry JA, Denmark HA, Campos CB (2004) A revised catalogue of the mite family Phytoseiidae. Zootaxa 434:1–494. https://doi.org/10.11646/zootaxa.434.1.1
Moral RA, Hinde J, Demétrio CGB (2017) Half-normal plots and overdispersed models in R: the hnp package. J Stat Softw 81:1–23. https://doi.org/10.18637/jss.v081.i10
Oliveira DGP, Pauli G, Mascarin GM, Delalibera I (2015) A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J Microbiol Methods 119:44–52. https://doi.org/10.1016/j.mimet.2015.09.021
Ownley BH, Griffin MR, Klingeman WE, Gwinn KD, Moulton JK, Pereira RM (2008) Beauveria bassiana: endophytic colonization and plant disease control. J Invertebr Pathol 3:267–270. https://doi.org/10.1016/j.jip.2008.01.010
Ownley B, Gwinn K, Vega F (2010) Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. Biocontrol 55:113–128. https://doi.org/10.1007/s10526-009-9241-x
Parsa S, Ortiz V, Vega FE (2013) Establishing fungal entomopathogens as endophytes: towards endophytic biological control. J Vis Exp 74:e50360. https://doi.org/10.3791/50360
Parsa S, Ortiz V, Gómez-Jiménez MI, Kramer M, Vega FE (2018) Root environment is a key determinant of fungal entomopathogen endophytism following seed treatment in the common bean, Phaseolus vulgaris. Biol Control 116:74–81. https://doi.org/10.1016/j.biocontrol.2016.09.001
Patiño-Ruiz JD, Schausberger P (2014) Spider mites adaptively learn recognizing mycorrhiza-induced changes in host plant volatiles. Exp Appl Acarol 64:455–463. https://doi.org/10.1007/s10493-014-9845-4
Quesada-Moraga E, Muñoz-Ledesma F, Santiago-Alvarez C (2009) Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ Entomol 38:723–730. https://doi.org/10.1603/022.038.0324
Raworth DA (1986) An economic threshold function for the twospotted spider mite, Tetranychus urticae (Acari: Tetranychidae), on strawberries. Can Entomol 118:9–16. https://doi.org/10.4039/Ent1189-1
Rehner SA (2005) Phylogenetics of the insect pathogenic genus Beauveria. In: Vega FE, Blackwell M (eds) Insect fungal associations: ecology and evolution, 1st edn. University Press, New York, pp 3–27
Rehner SA, Buckley EP (2005) A Beauveria phylogeny inferred from ITS and EF1-a sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98. https://doi.org/10.3852/mycologia.97.1.84
Rezende JM, Zanardo ABR, Lopes MD, Delalibera I Jr, Rehner SA (2015) Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. Biocontrol 60:495–505. https://doi.org/10.1007/s10526-015-9656-5
Rhodes EM, Liburd OE, Kelts C, Rondon SI, Francis RR (2006) Comparison of single and combination treatments of Phytoseiulus persimilis, Neoseiulus californicus, and Acramite (bifenazate) for control of twospotted spider mites in strawberries. Exp Appl Acarol 39:213–225. https://doi.org/10.1007/s10493-006-9005-6
Rowell HJ, Chant DA, Hansell R (1978) The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can Entomol 110(8):859–876. https://doi.org/10.4039/Ent110859-8
Sabbahi R, Merzouki A, Guertin C (2008) Efficacy of Beauveria bassiana against the strawberry pests, Lygus lineolaris, Anthonomus signatus and Otiorhynchus ovatus. J Appl Entomol 132:151–160. https://doi.org/10.1111/j.1439-0418.2007.01248.x
Sances FV, Wyman JA, Ting IP (1979) Physiological responses to spider mite infestation on strawberries. Environ Entomol 8:711–714. https://doi.org/10.1093/ee/8.4.711
Sances FV, Toscano NC, Oatman ER, Lapre LF, Johnson MW, Voth V (1982) Reductions in plant processes by Tetranychus urticae (Acari: Tetranychidae) feeding on strawberry. Environ Entomol 11:733–737. https://doi.org/10.1093/ee/11.3.733
Sánchez-Rodríguez AR, Raya-Díaz S, Zamarreño ÁM, García-Mina JM, Campillo MC, Quesada-Moraga E (2018) An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol Control 116:90–102. https://doi.org/10.1016/j.biocontrol.2017.01.012
Sasan RK, Bidochka MJ (2013) Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. sp. phaseoli. Can J Plant Pathol 35:288–293. https://doi.org/10.1080/07060661.2013.823114
Sato ME, Da Silva MZ, Raga A, De Souza Filho MF (2005) Abamectin resistance in Tetranychus urticae Koch (Acari Tetranychidae): selection, cross-resistance and stability of resistance. Neotrop Entomol 34:991–998. https://doi.org/10.1590/S1519-566X2005000600016
Schausberger P, Peneder S, Juerschik S, Hoffmann D (2012) Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct Ecol 26:441–449. https://doi.org/10.1111/j.1365-2435.2011.01947.x
Solomon MG, Jay CN, Innocenzi PJ, Fitzgerald JD, Crook D, Crook AM, Easterbrook MA, Cross JV (2001) Review: natural enemies and biocontrol of pests of strawberry in Northern and Central Europe. Biocontrol Sci Technol 11:165–216. https://doi.org/10.1080/09583150120035639
Stone JK, Polishook JD, White JRJ (2004) Endophytic fungi. In: Mueller G, Bills GF, Foster MS (eds) Biodiversity of fungi: inventory and monitoring method, 1st edn. Elsevier, USA, pp 241–270
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 2 Nov 2018
Tixier M-S, Guichou S, Kreiter S (2008) Morphological variation in the biological control agent Neoseiulus californicus (McGregor) (Acari: Phytoseiidae): consequences for diagnostic reliability and synonymies. Invertebr Syst 22:453–469. https://doi.org/10.1071/IS07052
Van Leeuwen T, Vontas J, Tsagkarakou A (2009) Mechanisms of acaricide resistance in the two spotted spider mite Tetranychus urticae. In: Ishaaya I, Horowitz AR (eds) Biorational control of arthropod pests, 1st edn. Springer, The Netherlands, pp 347–393
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauwa W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572. https://doi.org/10.1016/j.ibmb.2010.05.008
Vega FE (2008) Insect pathology and fungal endophytes. J Invertebr Pathol 98:277–279. https://doi.org/10.1016/j.jip.2008.01.008
Vega FE (2018) The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110:4–30. https://doi.org/10.1080/00275514.2017.1418578
Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Koike M, Maniania NK, Monzón A, Ownley BH, Pell JK, Rangel DEN, Roy HE (2009) Fungal entomopathogens: new insights on their ecology. Fungal Ecol 2:149–159. https://doi.org/10.1016/j.funeco.2009.05.001
Vidal S, Jaber LR (2015) Entomopathogenic fungi as endophytes: plant-endophyte-herbivore interactions and prospects for use in biological control. Curr Sci 109:46–54
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Yan J, Broughton S, Yang S, Gange A (2015) Do endophytic fungi grow through their hosts systemically? Fungal Ecol 13:53–59. https://doi.org/10.1016/j.funeco.2014.07.005
Acknowledgements
Daniela Milanez Silva and Vitor Isaias da Silva are thanked for technical assistance. We thank the strawberry producers Claudio Donizete dos Santos, Rafael Maziero, Mario Inui and Maurício dos Santos for letting us perform the experiments in their fields. We also thank Dr. Fagoni Fayer Calegario for helping to find the farmers and for introducing them to us. Dr. Geovanny Barroso is thanked for helping with the predatory mite identification.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) [Process No. 141373/2015-6] and by The Research Council of Norway through the SMARTCROP Project [Project Number 244526]. A 3-month student mission travel Grant to Norway was funded by CAPES (Project Number 88881.117865/2016-01) and SIU (Project Number UTF-2016-long-term-/10070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Quesada-Moraga.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Canassa, F., Esteca, F.C.N., Moral, R.A. et al. Root inoculation of strawberry with the entomopathogenic fungi Metarhizium robertsii and Beauveria bassiana reduces incidence of the twospotted spider mite and selected insect pests and plant diseases in the field. J Pest Sci 93, 261–274 (2020). https://doi.org/10.1007/s10340-019-01147-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01147-z