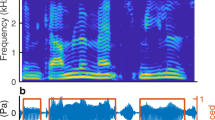
Abstract
An important topic in contemporary auditory science is supra-threshold hearing. Difficulty hearing at conversational speech levels in background noise has long been recognized as a problem of sensorineural hearing loss, including that associated with aging (presbyacusis). Such difficulty in listeners with normal thresholds has received more attention recently, especially associated with descriptions of synaptopathy, the loss of auditory nerve (AN) fibers as a result of noise exposure or aging. Synaptopathy has been reported to cause a disproportionate loss of low- and medium-spontaneous rate (L/MSR) AN fibers. Several studies of synaptopathy have assumed that the wide dynamic ranges of L/MSR AN fiber rates are critical for coding supra-threshold sounds. First, this review will present data from the literature that argues against a direct role for average discharge rates of L/MSR AN fibers in coding sounds at moderate to high sound levels. Second, the encoding of sounds at supra-threshold levels is examined. A key assumption in many studies is that saturation of AN fiber discharge rates limits neural encoding, even though the majority of AN fibers, high-spontaneous rate (HSR) fibers, have saturated average rates at conversational sound levels. It is argued here that the cross-frequency profile of low-frequency neural fluctuation amplitudes, not average rates, encodes complex sounds. As described below, this fluctuation-profile coding mechanism benefits from both saturation of inner hair cell (IHC) transduction and average rate saturation associated with the IHC-AN synapse. Third, the role of the auditory efferent system, which receives inputs from L/MSR fibers, is revisited in the context of fluctuation-profile coding. The auditory efferent system is hypothesized to maintain and enhance neural fluctuation profiles. Lastly, central mechanisms sensitive to neural fluctuations are reviewed. Low-frequency fluctuations in AN responses are accentuated by cochlear nucleus neurons which, either directly or via other brainstem nuclei, relay fluctuation profiles to the inferior colliculus (IC). IC neurons are sensitive to the frequency and amplitude of low-frequency fluctuations and convert fluctuation profiles from the periphery into a phase-locked rate profile that is robust across a wide range of sound levels and in background noise. The descending projection from the midbrain (IC) to the efferent system completes a functional loop that, combined with inputs from the L/MSR pathway, is hypothesized to maintain “sharp” supra-threshold hearing, reminiscent of visual mechanisms that regulate optical accommodation. Examples from speech coding and detection in noise are reviewed. Implications for the effects of synaptopathy on control mechanisms hypothesized to influence supra-threshold hearing are discussed. This framework for understanding neural coding and control mechanisms for supra-threshold hearing suggests strategies for the design of novel hearing aid signal-processing and electrical stimulation patterns for cochlear implants.











Similar content being viewed by others
References
Almishaal A, Jennings SG (2016) Effects of a precursor on amplitude modulation detection are consistent with efferent feedback. J Acoust Soc Am 139:2155–2155
Arnott RH, Wallace MN, Shackleton TM, Palmer AR (2004) Onset neurones in the anteroventral cochlear nucleus project to the dorsal cochlear nucleus. J Assoc Res Otolaryngol 5:153–170
Babalian AL, Ryugo DK, Vischer MW, Rouiller EM (1999) Inhibitory synaptic interactions between cochlear nuclei: evidence from an in vitro whole brain study. Neuroreport 10:1913–1917
Beattie RC, Raffin MJ (1985) Reliability of threshold, slope, and PB max for monosyllabic words. J Speech Hear Disord 50:166–178
Beattie RC, Edgerton BJ, Svihovec DV (1977) A comparison of the Auditec of St. Louis cassette recordings of NU-6 and CID W-22 on a normal-hearing population. J Speech Hear Disord 42:60–64
Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG (2014) Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci 8:26
Blackburn CC, Sachs MB (1990) The representations of the steady-state vowel sound /Ɛ/ in the discharge patterns of cat anteroventral cochlear nucleus neurons. J Neurophysiol 63:1191–1212
Cant NB (1993) The synaptic organization of the ventral cochlear nucleus of the cat: the peripheral cap of small cells. In: Merchán MA, Juiz JM, Godfrey DA, Mugnaini E (eds) The mammalian cochlear nuclei: organization and function. Springer, New York, pp 91–105
Cant NB (2005) Projections from the cochlear nuclear complex to the inferior colliculus. In: Winer JA, Schreiner CE (eds) The inferior colliculus. Springer, New York, pp 115–131
Cant NB, Benson CG (2003) Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 60:457–474
Cant NB, Oliver DL (2018) Overview of the organization of the mammalian auditory pathways: projection pathways and intrinsic microcircuits. In: Oliver DL, Cant NB, Fay RR, Popper AN (eds) The mammalian auditory pathways: synaptic organization and microcircuits. Springer handbook of auditory research. Springer, New York
Carlyon RP (1987) A release from masking by continuous, random, notched noise. J Acoust Soc Am 81:418–426
Carlyon R (1989) Changes in the masked thresholds of brief tones produced by prior bursts of noise. Hear Res 41:223–235
Carlyon RP, Moore BC (1984) Intensity discrimination: a severe departure from Weber’s law. J Acoust Soc Am 76:1369–1376
Carney LH (2018) Fluctuation contrast and speech-on-speech masking: model midbrain responses to simultaneous speech. In: Santurette S, Dau T, Dalsgaard JC, Tranebjærg L, Andersen T, Poulsen T (eds) Adaptive Processes in Hearing, Proceedings of the International Symposium on Auditory and Audiological Research (ISAAR), vol 6. The Danavox Jubilee Foundation, Ballerup, pp 75–82
Carney LH, Schwarz DM (2014) A Speech Enhancement Strategy based on Midbrain Response Properties, Abstract IHCON meeting.
Carney LH, Richards VM (2016) Predicting masked thresholds based on physiological models of auditory nerve and inferior colliculus population responses. J Acoust Soc Am 140:3272. https://doi.org/10.1121/1.4970387
Carney LH, Li T, McDonough JM (2015) Speech coding in the brain: representation of vowel formants by midbrain neurons tuned to sound fluctuations. eNeuro 2
Carney LH, Kim DO, Kuwada S (2016) Speech coding in the midbrain: effects of sensorineural hearing loss. In Physiology, psychoacoustics and cognition in normal and impaired hearing, Springer. Advances in experimental medicine and biology. 894:427–435
Carney LH, Oetjen H, Klump G (2017) Discrimination of Schroeder-phase complex stimuli: physiological, behavioral, and modeling studies in the Mongolian gerbil. J Acoust Soc Am 141:3827. https://doi.org/10.1121/1.4988494
Caspary DM, Ling L, Turner JG, Hughes LF (2008) Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211:1781–1791
Cheatham MA, Dallos P (2000) The dynamic range of inner hair cell and organ of Corti responses. J Acoust Soc Am 107:1508–1520
Colburn HS, Carney LH, Heinz MG (2003) Quantifying the information in auditory-nerve responses for level discrimination. J Assoc Res Otolaryngol 4:294–311
Cooper NP, Yates GK (1994) Nonlinear input-output functions derived from the responses of guinea-pig cochlear nerve fibres: variations with characteristic frequency. Hear Res 78:221–234
Dallos P (1985) Response characteristics of mammalian cochlear hair cells. J Neurosci 5:1591–1608
Dallos P (1986) Neurobiology of cochlear inner and outer hair cells: intracellular recordings. Hear Res 22:185–198
Dau T, Kollmeier B, Kohlrausch A (1997a) Modeling auditory processing of amplitude modulation. I. Detection and masking with narrow-band carriers. J Acoust Soc Am 102:2892–2905
Dau T, Kollmeier B, Kohlrausch A (1997b) Modeling auditory processing of amplitude modulation. II. Spectral and temporal integration. J Acoust Soc Am 102:2906–2919
Davis KA, Hancock KE, Delgutte B (2010) Computational models of inferior colliculus neurons. In: Meddis R, Lopez-Poveda E, Fay RR, Popper AN (eds) Computational models of the auditory system. Springer, New York, pp 129–176
Dean I, Harper NS, McAlpine D (2005) Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8:1684–1689
Delano PH, Elgueda D, Hamame CM, Robles L (2007) Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci 27:4146–4153
Delgutte B (1987) Peripheral auditory processing of speech information: implications from a physiological study of intensity discrimination. In: Schouten ME (ed) The psychophysics of speech perception. Springer, Netherlands, pp 333–353
Delgutte B (1996) Physiological models for basic auditory percepts. In: Hawkins HL, McMullen TA, Fay RR (eds) Auditory computation. Springer, New York, pp 157–220
Delgutte B, Kiang NY (1984a) Speech coding in the auditory nerve: I. Vowel-like sounds. J Acoust Soc Am 75:866–878
Delgutte B, Kiang NY (1984b) Speech coding in the auditory nerve: V. Vowels in background noise. J Acoust Soc Am 75:908–918
Deng L, Geisler CD (1987) Responses of auditory-nerve fibers to nasal consonant–vowel syllables. J Acoust Soc Am 82:1977–1988
Deng L, Geisler CD, Greenberg S (1987) Responses of auditory-nerve fibers to multiple-tone complexes. J Acoust Soc Am 82:1989–2000
Doucet JR, Ryugo DK (2006) Structural and functional classes of multipolar cells in the ventral cochlear nucleus. Anat Rec 288:331–344
Dragicevic CD, Aedo C, León A, Bowen M, Jara N, Terreros G, Robles L, Delano PH (2015) The olivocochlear reflex strength and cochlear sensitivity are independently modulated by auditory cortex microstimulation. J Assoc Res Otolaryngol 16:223–240
Encina-Llamas G, Aravindakshan P, Harte JM, Dau T, Kujawa SG, Shinn-Cunningham B, Epp B (2017) Hidden hearing loss with envelope following responses (EFRs): the off-frequency problem. ARO Abstracts 40:4
Fan L, Carney LH (2017) Neural responses in the inferior colliculus to diotic tone-in-noise stimuli support detection based on envelope and neural fluctuation, Abstract, Association for Research in Otolaryngology 40:250
Fant G (1960) Acoustic theory of speech perception. Mouton, The Hague
Fettiplace R, Ricci AJ (2006) Mechanoelectrical transduction in auditory hair cells. In: Eatock RA, Fay RR (eds) Vertebrate hair cells. Springer, New York, pp 154–203
Florentine M, Buus SR (1981) An excitation-pattern model for intensity discrimination. J Acoust Soc Am 70:1646–1654
Florentine M, Buus SR, Mason CR (1987) Level discrimination as a function of level for tones from 0.25 to 16 kHz. J Acoust Soc Am 81:1528–1541
Forrest TG, Green DM (1987) Detection of partially filled gaps in noise and the temporal modulation transfer function. J Acoust Soc Am 82:1933–1943
Freyman RL, Griffin AM, Oxenham AJ (2012) Intelligibility of whispered speech in stationary and modulated noise maskers. J Acoust Soc Am 132:2514–2523
Fuente A (2015) The olivocochlear system and protection from acoustic trauma: a mini literature review. Front Syst Neurosci 9(94):1–6
Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110:577–586
Gai Y, Carney LH (2006) Temporal measures and neural strategies for detection of tones in noise based on responses in anteroventral cochlear nucleus. J Neurophysiol 96:2451–2464
Gai Y, Carney LH (2008) Influence of inhibitory inputs on rate and timing of responses in the anteroventral cochlear nucleus. J Neurophysiol 99:1077–1095
Ghoshal S, Kim DO (1996) Marginal shell of the anteroventral cochlear nucleus: intensity coding in single units of the unanesthetized, decerebrate cat. Neurosci Lett 205:71–74
Green DM (1988) Profile analysis: auditory intensity discrimination (no. 13). Oxford University Press, Oxford
Guinan JJ (2011) Physiology of the medial and lateral olivocochlear systems. In: Ryugo DK, Fay RR, Popper AN (eds) Auditory and vestibular efferents. Springer, New York, pp 39–81
Guinan Jr JJ (1996) Physiology of olivocochlear efferents. In: Dallos P, Fay RR (eds) The cochlea. Springer, New York, pp 435–502
Gummer M, Yates GK, Johnstone BM (1988) Modulation transfer function of efferent neurones in the guinea pig cochlea. Hear Res 36:41–51
Harrison JM, Howe ME (1974) Anatomy of the afferent auditory nervous system of mammals. In: Keidel WD, Neff WD (eds) Auditory system. Springer, Berlin Heidelberg, pp 283–336
Heinz MG, Colburn HS, Carney LH (2001a) Evaluating auditory performance limits: I. One-parameter discrimination using a computational model for the auditory nerve. Neural Comput 13:2273–2316
Heinz MG, Colburn HS, Carney LH (2001b) Rate and timing cues associated with the cochlear amplifier: level discrimination based on monaural cross-frequency coincidence detection. J Acoust Soc Am 110:2065–2084
Helmholtz HV (1909) In: JPC S (ed) Physiological optics, vol 3. Optical Society of America, Washington, DC
Henry KS, Kale S, Heinz MG (2014) Noise-induced hearing loss increases the temporal precision of complex envelope coding by auditory-nerve fibers. Front Syst Neurosci 8:1–10
Henry KS, Abrams KS, Forst J, Mender MJ, Neilans EG, Idrobo F, Carney LH (2017) Midbrain synchrony to envelope structure supports behavioral sensitivity to single-formant vowel-like sounds in noise. J Assoc Res Otolaryngol 18:165–181
Hienz RD, Stiles P, May BJ (1998) Effects of bilateral olivocochlear lesions on vowel formant discrimination in cats. Hear Res 116:10–20
Hillenbrand J, Getty LA, Clark MJ, Wheeler K (1995) Acoustic characteristics of American English vowels. J Acoust Soc Am 97:3099–3111
Hirsh IJ, Davis H, Silverman SR, Reynolds EG, Eldert E, Benson RW (1952) Development of materials for speech audiometry. Journal of Speech and Hearing Disorders 17:321–337
Huet A, Batrel C, Tang Y, Desmadryl G, Wang J, Puel JL, Bourien J (2016) Sound coding in the auditory nerve of gerbils. Hear Res 338:32–39
Huffman RF, Henson OW (1990) The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Rev 15:295–323
Jacewicz E, Fox RA, Salmons J (2007) Vowel duration in three American English dialects. American Speech 82:367–385
Jackson BS, Carney LH (2005) The spontaneous-rate histogram of the auditory nerve can be explained by only two or three spontaneous rates and long-range dependence. J Assoc Res Otolaryngol 6:148–159
Jenkins WM, Masterton RB (1982) Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol 47:987–1016
Jennings SG, Strickland EA, Heinz MG (2009) Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking. J Acoust Soc Am 125:2172–2181
Jesteadt W, Schairer KS, Neff DL (2005) Effect of variability in level on forward masking and on increment detection. J Acoust Soc Am 118:325–337
Johnson DH (1980) The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am 68:1115–1122
Joris PX (2003) Interaural time sensitivity dominated by cochlea-induced envelope patterns. J Neurosci 23:6345–6350
Joris PX, Yin TC (1992) Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am 91:215–232
Joris PX, Schreiner CE, Rees A (2004) Neural processing of amplitude-modulated sounds. Physiol Rev 84:541–577
Kale S, Heinz MG (2010) Envelope coding in auditory nerve fibers following noise-induced hearing loss. J Assoc Res Otolaryngol 11:657–673
Kidd Jr G, Mason CR, Brantley MA, Owen GA (1989) Roving-level tone-in-noise detection. J Acoust Soc Am 86:1310–1317
Kim DO, Zahorik P, Carney LH, Bishop BB, Kuwada S (2015) Auditory distance coding in rabbit midbrain neurons and human perception: monaural amplitude modulation depth as a cue. J Neurosci 35:5360–5372
Kohlrausch A, Püschel D, Alphei H (1992) Temporal resolution and modulation analysis in models of the auditory system. The Auditory Processing of Speech: From Sounds to Words 10:85–98
Kohlrausch A, Fassel R, van der Heijden M, Kortekaas R, van de Par S, Oxenham AJ, Püschel D (1997) Detection of tones in low-noise noise: further evidence for the role of envelope fluctuations. Acta Acustica United with Acustica 83:659–669
Krishna BS, Semple MN (2000) Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol 84:255–273
Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29:14077–14085
Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199
Langner G, Schreiner CE (1988) Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. J Neurophysiol 60:1799–1822
Leake PA, Snyder RL (1989) Topographic organization of the central projections of the spiral ganglion in cats. J Comp Neurol 281:612–629
Lentz JJ, Richards VM, Matiasek MR (1999) Different auditory filter bandwidth estimates based on profile analysis, notched noise, and hybrid tasks. J Acoust Soc Am 106:2779–2792
Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63:442–455
Liberman MC (1991) Central projections of auditory-nerve fibers of differing spontaneous rate. I. Anteroventral cochlear nucleus. J Comp Neurol 313:240–258
Liberman MC, Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 349:138–147. https://doi.org/10.10106/j.heares.2017.01.003
Lobarinas E, Salvi R, Ding D (2013) Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res 302:113–120
Lopez-Poveda EA (2014) Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Front Neurosci 8:1–7
Lopez-Poveda EA, Eustaquio-Martín A (2006) A biophysical model of the inner hair cell: the contribution of potassium currents to peripheral auditory compression. J Assoc Res Otolaryngol 7:218–235
Lowen SB, Teich MC (1996) The periodogram and Allan variance reveal fractal exponents greater than unity in auditory-nerve spike trains. J Acoust Soc Am 99:3585–3591
Lyzenga J, Horst JW (1997) Frequency discrimination of stylized synthetic vowels with a single formant. J Acoust Soc Am 102:1755–1767
Maison S, Micheyl C, Collet L (1999) Sinusoidal amplitude modulation alters contralateral noise suppression of evoked otoacoustic emissions in humans. Neuroscience 91:133–138
Manis PB, Xie R, Wang Y, Marrs GS, Spirou GA (2012) The endbulbs of held. In: Trussell L, Popper A, Fay R (eds) Synaptic mechanisms in the auditory system. Springer handbook of auditory research, vol 41. Springer, New York, pp 61–93
Mao J, Carney LH (2015) Tone-in-noise detection using envelope cues: comparison of signal-processing-based and physiological models. J Assoc Res Otolaryngol 16:121–133
Mao J, Vosoughi A, Carney LH (2013) Predictions of diotic tone-in-noise detection based on a nonlinear optimal combination of energy, envelope, and fine-structure cues. J Acoust Soc Am 134:396–406
May BJ, McQuone SJ (1995) Effects of bilateral olivocochlear lesions on pure-tone intensity discrimination in cats. Audit Neurosci 1:385–400
May BJ, Sachs MB (1992) Dynamic range of neural rate responses in the ventral cochlear nucleus of awake cats. J Neurophysiol 68:1589–1602
May BJ, Budelis J, Niparko JK (2004) Behavioral studies of the olivocochlear efferent system: learning to listen in noise. Arch Otolaryngol Head Neck Surg 130:660–664.
McGill WJ, Goldberg JP (1968) A study of the near-miss involving Weber’s law and pure-tone intensity discrimination. Atten Percept Psychophys 4:105–109
Mellott JG, Bickford ME, Schofield BR (2014) Descending projections from auditory cortex to excitatory and inhibitory cells in the nucleus of the brachium of the inferior colliculus. Front Syst Neurosci 8(188):1–15
Micheyl C, Maison S, Carlyon RP, Andéol G, Collet L (1999) Contralateral suppression of transiently evoked otoacoustic emissions by harmonic complex tones in humans. J Acoust Soc Am 105:293–305
Miller RL, Schilling JR, Franck KR, Young ED (1997) Effects of acoustic trauma on the representation of the vowel /ε/ in cat auditory nerve fibers. J Acoust Soc Am 101:3602–3616
Millman RE, Mattys SL, Gouws AD, Prendergast G (2017) Magnified neural envelope coding predicts deficits in speech perception in noise. J Neurosci 37(32):7727–7736. https://doi.org/10.1523/JNEUROSCI.2722-16
Moore BC (2012) An introduction to the psychology of hearing. Brill, Leiden
Moore BC, Glasberg BR (1993) Simulation of the effects of loudness recruitment and threshold elevation on the intelligibility of speech in quiet and in a background of speech. J Acoust Soc Am 94:2050–2062
Nayagam DA, Clarey JC, Paolini AG (2005) Powerful, onset inhibition in the ventral nucleus of the lateral lemniscus. J Neurophysiol 94:1651–1654
Nelson PC, Carney LH (2004) A phenomenological model of peripheral and central neural responses to amplitude-modulated tones. J Acoust Soc Am 116(4):2173–2186
Nelson PC, Carney LH (2007) Neural rate and timing cues for detection and discrimination of amplitude-modulated tones in the awake rabbit inferior colliculus. J Neurophysiol 97:522–539
Nilsson M, Soli SD, Sullivan JA (1994) Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am 95:1085–1099
Oertel D, Wu SH, Garb MW, Dizack C (1990) Morphology and physiology of cells in slice preparations of the posteroventral cochlear nucleus of mice. J Comp Neurol 295:136–154
Olsen WO (1998) Average speech levels and spectra in various speaking/listening conditions: a summary of the Pearson, Bennett, & Fidell (1977) report. Am J Audiol 7:21–25
Oxenham AJ (2016) Predicting the perceptual consequences of hidden hearing loss. Trends in Hearing 20:1–6
Oxenham AJ, Wojtczak M (2016) Predicting effects of hidden hearing loss using signal detection theory. J Acoust Soc Am 140:3150–3150
Palmer AR, Wallace MN, Arnott RH, Shackleton TM (2003) Morphology of physiologically characterised ventral cochlear nucleus stellate cells. Exp Brain Res 153:418–426
Patterson RD, Moore BCJ (1986) Auditory filters and excitation patterns as representations of frequency resolution. In: Moore BCJ (ed) Frequency selectivity in hearing. Academic, London, pp 123–177
Pickett JM (1956) Effects of vocal force on the intelligibility of speech sounds. J Acoust Soc Am 28:902–905
Pierscionek BK (1993) What we know and understand about presbyopia. Clin Exp Optom 76:83–90
Plack CJ, Barker D, Prendergast G (2014) Perceptual consequences of “hidden” hearing loss. Trends Hear 18:1–11
Plack CJ, Léger A, Prendergast G, Kluk K, Guest H, Munro KJ (2016) Toward a diagnostic test for hidden hearing loss. Trends Hear 20:1–9
Rao A, Carney LH (2014) Speech enhancement for listeners with hearing loss based on a model for vowel coding in the auditory midbrain. IEEE Trans Biomed Eng 61:2081–2091
Rees A, Langner G (2005) Temporal coding in the auditory midbrain. In: Winer JA, Schreiner CE (eds) The inferior colliculus. Springer, New York, pp 346–376
Remez RE, Rubin PE, Pisoni DB, Carrell TD (1981) Speech perception without traditional speech cues. Science 212:947–949
Rhode WS, Greenberg S (1992) Physiology of the cochlear nuclei. In: Popper AN, Fay RR (eds) The mammalian auditory pathway: neurophysiology. Springer, New York, pp 94–152
Rhode WS, Greenberg S (1994) Encoding of amplitude modulation in the cochlear nucleus of the cat. J Neurophysiol 71:1797–1825
Rhode WS, Oertel D, Smith PH (1983) Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat ventral cochlear nucleus. J Comp Neurol 213:448–463
Richards VM (1992) The detectability of a tone added to narrow bands of equal-energy noise. J Acoust Soc Am 91:3424–3435
Roberts WM, Rutherford MA (2008) Linear and nonlinear processing in hair cells. J Exp Biol 211:1775–1780
Ruggles DR, Freyman RL, Oxenham AJ (2014) Influence of musical training on understanding voiced and whispered speech in noise. PLoS One 9:e86980
Russell IJ, Sellick PM (1983) Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol 338:179–206
Russell IJ, Richardson GP, Cody AR (1986) Mechanosensitivity of mammalian auditory hair cells in vitro. Nature 321:517–519
Ryugo DK (2008) Projections of low spontaneous rate, high threshold auditory nerve fibers to the small cell cap of the cochlear nucleus in cats. Neuroscience 154:114–126
Ryugo DK, Sento S (1991) Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. J Comp Neurol 305:35–48
Sachs MB, Young ED (1979) Encoding of steady-state vowels in the auditory nerve: representation in terms of discharge rate. J Acoust Soc Am 66:470–479
Sachs MB, Voigt HF, Young ED (1983) Auditory nerve representation of vowels in background noise. J Neurophysiol 50:27–45
Sachs MB, Bruce IC, Miller RL, Young ED (2002) Biological basis of hearing-aid design. Ann Biomed Eng 30:157–168
Sachs MB, May BJ, Le Prell GS, Hienz RD (2006) Adequacy of auditory-nerve rate representations of vowels: comparison with behavioral measures in cat. In: Greenberg S, Ainsworth W (eds) Listening to speech: an auditory perspective. Lawrence Erlbaum, Mahwah, pp 115–127
Schaette R, McAlpine D (2011) Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31:13452–13457
Schalk TB, Sachs MB (1980) Nonlinearities in auditory-nerve fiber responses to bandlimited noise. J Acoust Soc Am 67:903–913
Schofield BR (2011) Central descending auditory pathways. In: Ryugo DK, Fay RR, Popper AN (eds) Auditory and vestibular Efferents. Springer, New York, pp 261–290
Schofield BR, Cant NB (1997) Ventral nucleus of the lateral lemniscus in guinea pigs: cytoarchitecture and inputs from the cochlear nucleus. J Comp Neurol 379:363–385
Schofield BR, Cant NB (1999) Descending auditory pathways: projections from the inferior colliculus contact superior olivary cells that project bilaterally to the cochlear nuclei. J Comp Neurol 409:210–223
Sewell WF (1984) Furosemide selectively reduces one component in rate-level functions from auditory-nerve fibers. Hear Res 15:69–72
Sherman SM, Spear PD (1982) Organization of visual pathways in normal and visually deprived cats. Physiol Rev 62:738–855
Siebert WM (1965) Some implications of the stochastic behavior of primary auditory neurons. Kybernetika 2:206–215
Sinex DG (2008) Responses of cochlear nucleus neurons to harmonic and mistuned complex tones. Hear Res 238:39–48
Sinex DG, Sabes JH, Li H (2002) Responses of inferior colliculus neurons to harmonic and mistuned complex tones. Hear Res 168:150–162
Sinex DG, Guzik H, Li H, Sabes JH (2003) Responses of auditory nerve fibers to harmonic and mistuned complex tones. Hear Res 182:130–139
Smith RL, Brachman ML (1982) Adaptation in auditory-nerve fibers: a revised model. Biol Cybern 44:107–120
Smith DW, Keil A (2015) The biological role of the medial olivocochlear efferents in hearing: separating evolved function from exaptation. Front Syst Neurosci 9(12):1–6
Smith PH, Rhode WS (1989) Structural and functional properties distinguish two types of multipolar cells in the ventral cochlear nucleus. J Comp Neurol 282:595–616
Smith RL, Zwislocki JJ (1975) Short-term adaptation and incremental responses of single auditory-nerve fibers. Biol Cybern 17:169–182
Smith RL, Brachman ML, Frisina RD (1985) Sensitivity of auditory-nerve fibers to changes in intensity: a dichotomy between decrements and increments. J Acoust Soc Am 78:1310–1316
Smith PH, Massie A, Joris PX (2005) Acoustic stria: anatomy of physiologically characterized cells and their axonal projection patterns. J Comp Neurol 482:349–371
Spirou GA, Brownell WE, Zidanic M (1990) Recordings from cat trapezoid body and HRP labeling of globular bushy cell axons. J Neurophysiol 63:1169–1190
Spirou GA, Rager J, Manis PB (2005) Convergence of auditory-nerve fiber projections onto globular bushy cells. Neuroscience 136:843–863
Strickland EA (2008) The relationship between precursor level and the temporal effect. J Acoust Soc Am 123:946–954
Strickland EA, Krishnan LA (2005) The temporal effect in listeners with mild to moderate cochlear hearing impairment. J Acoust Soc Am 118:3211–3217
Studebaker GA, Sherbecoe RL, McDaniel DM, Gwaltney CA (1999) Monosyllabic word recognition at higher-than-normal speech and noise levels. J Acoust Soc Am 105:2431–2444
Swaminathan J, Goldsworthy RL, Zurek PM, Léger AC, Braida LD (2014) Preliminary evaluation of a physiologically inspired signal processing strategy for cochlear implants. J Acoust Soc Am 135:2410–2410
Teich MC, Lowen SB (1994) Fractal patterns in auditory nerve-spike trains. IEEE Engineering in Medicine and Biology Magazine 13:197–202
Teich MC, Johnson DH, Kumar AR, Turcott RG (1990) Rate fluctuations and fractional power-law noise recorded from cells in the lower auditory pathway of the cat. Hear Res 46:41–52
Terreros G, Delano PH (2015) Corticofugal modulation of peripheral auditory responses. Front Syst Neurosci 9(134):1–8
Thompson AM, Thompson GC (1993) Relationship of descending inferior colliculus projections to olivocochlear neurons. J Comp Neurol 335:402–412
Tsuji J, Liberman MC (1997) Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol 381:188–202
Umeda N (1977) Consonant duration in American English. J Acoust Soc Am 61:846–858
van der Heijden M, Kohlrausch A (1995) The role of envelope fluctuations in spectral masking. J Acoust Soc Am 97:1800–1807
Victor JD, Nirenberg S (2013) Spike trains as event sequences: fundamental implications. In: DiLorenzo PM, Victor JD (eds) Spike timing: mechanisms and function. CRC Press, Boca Raton, pp 3–33
Viemeister NF (1988) Intensity coding and the dynamic range problem. Hear Res 34:267–274
Warr WB (1992) Organization of olivocochlear efferent systems in mammals. In: Webster DB, Fay R (eds) The mammalian auditory pathway: neuroanatomy. Springer, New York, pp 410–448
Warren EH, Liberman MC (1989) Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res 37:89–104
Weale RA (1962) Presbyopia. Br J Ophthalmol 46:660–668
Wen B, Wang GI, Dean I, Delgutte B (2009) Dynamic range adaptation to sound level statistics in the auditory nerve. J Neurosci 29:13797–13808
Wilson BS, Dorman MF (2008) Cochlear implants: a remarkable past and a brilliant future. Hear Res 242:3–21
Winslow RL, Sachs MB (1988) Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear Res 35:165–189
Winslow RL, Barta PE, Sachs MB (1987) Rate coding in the auditory nerve. In: Yost WA, Watson CS (eds) Auditory processing of complex sounds. Lawrence Erlbaum, Hillsdale, pp 212–224
Winter IM, Palmer AR (1991) Intensity coding in low-frequency auditory-nerve fibers of the guinea pig. J Acoust Soc Am 90:1958–1967
Winter IM, Robertson D, Yates GK (1990) Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibres. Hear Res 45:191–202
Wong S, Henry KS (2018) Effects of auditory-nerve damage on behavioral tone detection by budgerigars in quiet and in noise. ARO Abstracts 41:13
Yates GK (1990) Basilar membrane nonlinearity and its influence on auditory nerve rate-intensity functions. Hear Res 50:145–162
Yates G, Johnstone B, Patuzzi R, Robertson D (1992) Mechanical preprocessing in the mammalian cochlea. Trends Neurosci 15(2):57–61
Ye Y, Machado DG, Kim DO (2000) Projection of the marginal shell of the anteroventral cochlear nucleus to olivocochlear neurons in the cat. J Comp Neurol 420:127–138
Young ED, Davis KA (2002) Circuitry and function of the dorsal cochlear nucleus. In: Oertel D, Fay RR, Popper AN (eds) Integrative functions in the mammalian auditory pathway. Springer, New York, pp 160–206
Zeddies DG, Siegel JH (2004) A biophysical model of an inner hair cell. J Acoust Soc Am 116:426–441
Zhong Z, Henry KS, Heinz MG (2014) Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hear Res 309:55–62
Zilany MSA, Bruce IC (2007) Representation of the vowel /ε/ in normal and impaired auditory nerve fibers: model predictions of responses in cats. J Acoust Soc Am 122:402–417
Zilany MSA, Carney LH (2010) Power-law dynamics in an auditory-nerve model can account for neural adaptation to sound-level statistics. J Neurosci 30:10380–10390
Zilany MS, Bruce IC, Nelson PC, Carney LH (2009) A phenomenological model of the synapse between the inner hair cell and auditory nerve: long-term adaptation with power-law dynamics. J Acoust Soc Am 126:2390–2412
Zilany MS, Bruce IC, Carney LH (2014) Updated parameters and expanded simulation options for a model of the auditory periphery. J Acoust Soc Am 135:283–286
Zwicker E (1965) Temporal effects in simultaneous masking by white‐noise bursts. J Acoust Soc Am 37:653–663
Acknowledgements
The writing of this review was supported by NIH-DC-R01-001641, NIH-DC-R01-010813, by a sabbatical fellowship at Hanse Wissenschaftskolleg, Delmenhorst, Germany, and further inspired by conversations with colleagues in the Hearing Systems Group during a sabbatical visit to Technical University of Denmark. The manuscript was improved by helpful comments from Drs. David Cameron, Kenneth Henry, Joseph C. Holt, Brian Madden, Virginia Richards, Elizabeth Strickland, and the reviewers and by the editorial guidance of Dr. George Spirou.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carney, L.H. Supra-Threshold Hearing and Fluctuation Profiles: Implications for Sensorineural and Hidden Hearing Loss. JARO 19, 331–352 (2018). https://doi.org/10.1007/s10162-018-0669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-018-0669-5