Abstract
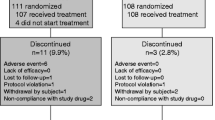
The aim of this study was to investigate the efficacy of concurrent treatment with vitamin K2 and risedronate compared with treatment with risedronate alone in patients with osteoporosis and to explore subsets of patients for which concurrent treatment is particularly efficacious. Women with osteoporosis aged 65 years or older were recruited from 123 institutes in Japan and allocated to take either vitamin K2 (45 mg/day) and risedronate (2.5 mg/day or 17.5 mg/week) or risedronate (2.5 mg/day or 17.5 mg/week) alone. The primary end point was the incidence of any fracture (vertebral and nonvertebral). The secondary end points were bone mineral density, height, undercarboxylated osteocalcin concentration, quality of life, and safety. Over a 2-year follow-up, vertebral or nonvertebral fractures occurred in 117 or 22 sites respectively among 931 patients in the risedronate and vitamin K2 group and in 104 or 26 sites respectively among 943 patients in the risedronate alone group. The rates of any incident fracture were similar between the two groups (incidence rate ratio 1.074, 95 % confidence interval 0.811–1.422, p = 0.62), implying that the primary end point was not met. There were no differences in the degree of increase in bone mineral density between the two groups. Undercarboxylated osteocalcin concentration decreased from 5.81 ± 3.93 ng/mL to 2.59 ± 1.52 ng/mL at 6 months in the risedronate and vitamin K2 group, whereas the change in the risedronate alone group was minimal (from 5.96 ± 4.36 ng/mL to 4.05 ± 3.40 ng/mL at 6 months) (p < 0.01). The treatment discontinuation rate was higher in the risedronate and vitamin K2 group than in the risedronate alone group (10.0 % vs 6.7 %). No unknown adverse drug reactions were reported. In conclusion, concurrent treatment with vitamin K2 and risedronate was not efficacious compared with monotherapy with risedronate in terms of fracture prevention.



Similar content being viewed by others
References
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–589
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 348:1535–1541
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Glüer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Carmel AS, Shieh A, Bang H, Bockman RS (2012) The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/mL. Osteoporos Int 23:2479–2487
Shiraki M, Yamazaki Y, Shiraki Y, Hosoi T, Tsugawa N, Okano T (2010) High level of serum undercarboxylated osteocalcin in patients with incident fractures during bisphosphonate treatment. J Bone Miner Metab 28:578–584
Shiraki M, Tsugawa N, Okano T (2015) Recent advances in vitamin K-dependent Gla-containing proteins and vitamin K nutrition. Osteoporos Sarcopenia 1:22–38
Shiraki M, Shiraki Y, Aoki C, Miura M (2000) Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 15:515–521
Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse R (2008) Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 5:e196
Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K (2008) Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 283:11270–11279
Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278(45):43919–43927
Tanaka S, Miyazaki T, Uemura Y, Kuroda T, Miyakawa N, Nakamura T, Fukunaga M, Ohashi Y, Ohta H, Mori S, Hagino H, Hosoi T, Sugimoto T, Itoi E, Orimo H, Shiraki M (2014) Design of a randomized clinical trial of concurrent treatment with vitamin K2 and risedronate compared to risedronate alone in osteoporotic patients: Japanese Osteoporosis Intervention Trial-03 (JOINT-03). J Bone Miner Metab 32:298–304
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. Osteoporosis Diagnostic Criteria Review Committee: Japanese Society for Bone and Mineral Research. J Bone Miner Metab 19:331–337
Orimo H, Nakamura T, Fukunaga M, Ohta H, Hosoi T, Uemura Y, Kuroda T, Miyakawa N, Ohashi Y, Shiraki M (2011) Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: the Japanese Osteoporosis Intervention Trial (JOINT)—02. Curr Med Res Opin 27:1273–1284
Kumamoto K, Nakamura T, Suzuki T, Gorai I, Fujinawa O, Ohta H, Shiraki M, Yoh K, Fujiwara S, Endo N, Matsumoto T (2010) Validation of the Japanese Osteoporosis Quality of Life Questionnaire. J Bone Miner Metab 28:1–7
Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A, Inoue T, Kaneda K, Morii H, Nawata H, Yamamoto K, Ohashi Y, Orimo H (2004) A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab 22:469–478
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2009) High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutr Res 29:221–228
Kushima Y, Ishii K, Shimizu K, Sasaki A, Yamamoto K et al (2007) Effect of combination therapy with risedronate and vitamin K2 for osteoporosis: multicentre collaborative study. Preliminary report of a 1-year study. Osteoporos Jpn 15:234–237
Fang Y, Hu C, Tao X, Wan Y, Tao F (2012) Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab 30:60–68
Stevenson M, Lloyd-Jones M, Papaioannou D (2009) Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health Technol Assess 13:1–134
Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Nakamura T, Sato T, Yamazaki K (2009) Randomized controlled study on the prevention of osteoporotic fractures (of study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab 27:66–75
Kasukawa Y, Miyakoshi N, Ebina T, Aizawa T, Hongo M, Nozaka K, Ishikawa Y, Saito H, Chida S, Shimada Y (2014) Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated osteocalcin and osteocalcin levels in postmenopausal osteoporosis. J Bone Miner Metab 32:290–297
Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17:315–321
Acknowledgments
The authors thank the leading investigators involved in this study at the following medical facilities: Yoshida Orthopedic Clinic, Mie Memorial Hospital, Sapporo Kiyota Orthopedic Hospital, Irei Seikeigeka, and National Hospital Organization Fukui Hospital. Thanks are extended to all investigators involved in this study at the following medical facilities: Ako Hakuhou Hospital, Akiyama Orthopedic Clinic, Arai Clinic, Chiba Foundation for Health Promotion and Disease Prevention, Chousei Hospital, Fujii Internal Clinic, Fujisaki Orthopedic Clinic, Fujiwara Memorial Hospital, Fukase Orthopedic Clinic, Fuki City Hospital, Fukujukai Clinic, Fukui Kousei Hospital, Fukuroi City Hospital (integrated), Fukuroku Orthopedic Clinic, Fukutoku Orthopedic Surgery Clinic, Gomyo Orthopedic Clinic, Gyoda General Hospital, Hakodate Red Cross Hospital, Hakodate Central Hospital, Hara Orthopedic Clinic, Haramoto Clinic, Hayashi Orthopedic Clinic, Hayashi Surgery Clinic, Hirose Clinic, Hitachi Yokohama Hospital, Ichinomiya-nishi Hospital (closed), Inabe General Hospital, Ishida Orthopedic Clinic, JA Gifu Tohno Kosei Hospital, Japanese Red Cross Kyoto Daini Hospital, Japanese Red Cross Nagoya Daini Hospital, Joto Central Hospital Orthopedic Clinic, Kamiyouga Setagaya Street Clinic, Katsuura Orthopedic Clinic, Katsuga Orthopedic Clinic, Kawashima Orthopedic Hospital/Kawasima Clinic, Kenmochi Clinic, Kitasato University Hospital, Kodama Clinic, Takano Geka Seikeigeka, Kurosawa Hospital, Kurume University Hospital, Kyoto City Hospital, Kyoto Katsura Hospital, Matsui Orthopedic Clinic, Murase Hospital, Matsukuchi Orthopedic Clinic, Matsuzaka Central General Hospital, Miyazaki Orthopedic Clinic, Mori Orthopedic Clinic, Minami Hospital, Morooka Orthopedic Clinic, Nashinoki Clinic, National Hospital Organization Utano Hospital, National Hospital Organization Miyazaki Higashi Hospital, Nishimura Orthopedic Clinic, Nozaki Clinic, Ota City Hospital, Ota Orthopedic Clinic, Ootaki Hospital, Otoda Orthopedic Internal Clinic, Okumura Orthopedic Clinic, Ota General Hospital, Obase Hospital, Orito Orthopedic Clinic, Osaka City University Hospital, Research Institute and Practice for lnvolutional Disease, Saiseikai Kawaguchi General Hospital, Sanada Clinic, Sakura Clinic, Sappro Orthopedic Clinic, Saiseikai Matsuyama Hospital, Saito Orthopedic Clinic, Seirei Hamamatsu Hospital, Senkawa-Shinoda Orthopedic Clinic, Shibata Clinic, Shiozawa Orthopedic Clinic, Shinseikai Hospital, Shin Sapporo Orthopedic Hospital, Soejima Orthopedic Clinic, Suga Surgical Orthopedic Clinic, Sugioka Orthopedic Clinic, Takahashi Orthopedic Clinic, Takakuwa Orthopedic Nagayama Clinic, Tokyo Medical and Dental University, University Hospital of Medicine, Tottori University School of Medicine, Tokyo Women’s Medical University Medical Center Obstetrics and Gynecology, Toyokawa City Hospital, Tsuji Clinic, Tsukabara Clinic, Tsuruga City Hospital, Yamao Clinic, Yamane Hospital, Yasue Hospital, Yamamoto Orthopedic Clinic, Yanagisawa Orthopedic Clinic, Yodakubo National Health Insurance Hospital, Ui Orthopedic Clinic, Ugaji Orthopedic Clinic, Unnan City Hospital, University of Toyama Hospital, Wada Women’s Clinic, Watanabe Clinic, Zenshukai Hospital, Toyama Prefecture Saiseikai Takaoka Hospital, Shibayama Seikeigeka, Makiyama Central Hospital, Toumi City Mimaki Spa Clinic, Okuyama Orthopedic Clinic, Yoshida Clinic, Hamazaki Clinic, Tama-sougo Hospital (closed), Noda Clinic, Furuoka Orthopedic Clinic, Iseikai Hospital, and Murakami Orthopedic Clinic. The authors also express thanks to the chairman (Rikushi Morita) and the members of the Ethics Committee: Mamiko Matsumura, Tetsuro Inoue, Isao Yoshimura, Mitsuyoshi Nakajima (died), Tooru Ebihara, Shinya Hattori, and Kousaku Uchida. The authors also express sincere thanks to members of the A-TOP research group: Shigeto Morimoto (Geriatric Medicine, Kanazawa Medical University), Akira ltabashi (Saitama Center for Bone Research, Kubojima Clinic), Toshihiko Yamashita (formerly of the Department of Orthopedic Surgery, Sapporo Medical University School of Medicine), Hideaki Kishimoto (Department of Orthopedic Surgery, San-in Rosai Hospital), Hideki Mizunurna (Department of Obstetrics and Gynecology, Hirosaki University School of Medicine), Naoto Endo (Division of Orthopedic Surgery, Niigata University Medical and Dental Hospital), Yoshiki Nishizawa (formerly of the Department of Metabolism, Endocrinology, and Molecular Medicine, Osaka City University Graduate School of Medicine), and Kunio Takaoka (formerly of the Department of Orthopedic Surgery, Osaka City University Medical School) for their help in establishing the study design, and Naoto Fujinawa (I’cros), Kazumasa Tanzawa (formerly of ING Corporation), and Akan Katagiri (Japanese Clinical Research Support Unit) for their contribution to data management. Japanese Osteoporosis Intervention Trial-03 was sponsored by the Public Health Research Foundation. The authors also thank Eisai, Teijin Pharma Asahi Kasei, Takeda Pharmaceutical, GE Healthcare Japan, Toyo Medic, Banyu Pharmaceutical, and Dainippon Sumitomo Pharma for their generous donations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Teruhiko Miyazaki is an employee of the Public Health Research Foundation. All other authors have no conflicts of interest.
About this article
Cite this article
Tanaka, S., Miyazaki, T., Uemura, Y. et al. Comparison of concurrent treatment with vitamin K2 and risedronate compared with treatment with risedronate alone in patients with osteoporosis: Japanese Osteoporosis Intervention Trial-03. J Bone Miner Metab 35, 385–395 (2017). https://doi.org/10.1007/s00774-016-0768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-016-0768-5