Abstract
Summary
This study compares efficacy of ALN/D5600 versus that of calcitriol in osteoporotic Chinese postmenopausal women. ALN/D5600 produced greater bone mineral density (BMD) increases, greater bone turnover marker decreases, and less vitamin D insufficiency. This study provided detailed clinical information regarding ALN/D5600 treatment versus calcitriol 0.25 μg/day. The study did not evaluate fracture risk.
Introduction
The aim of this study is to investigate efficacy of alendronate 70 mg/vitamin D3 5600 IU combination tablets (ALN/D5600) versus calcitriol in osteoporotic Chinese postmenopausal women.
Methods
This study is a 6-month, randomized, open-label, active-comparator study with 6-month extension (clinicaltrials.gov number NCT01350934) in postmenopausal women aged >55 years with osteoporosis (low bone mineral density (BMD) with/without prior fragility fracture). Patients were randomized to ALN/D5600 once weekly or calcitriol 0.25 μg daily. The primary efficacy end point of the base study was percent change from baseline in lumbar spine BMD (month 6). Hypercalcemia and hypercalciuria were safety events of special interest.
Results
A total of 219 patients (ALN/D5600 n = 111, calcitriol n = 108) were randomized. Baseline characteristics were similar, 30.3 % baseline 25-hydroxyvitamin D (25(OH)D) ≤15 ng/mL. At months 6 and 12, changes in lumbar spine BMD from baseline were 3.5 versus 1.6 % and 5.2 versus 2.3 % for ALN/D5600 versus calcitriol (between-group differences p < 0.001), respectively. Between-group differences for ALN/D5600 versus calcitriol were significant (p < 0.001) at months 6 and 12 for change from baseline in procollagen type 1 N-terminal propeptide (−59.1 versus −16.8 %, −68.1 versus −17.0 %) and serum C-telopeptides (−79.2 versus −27.2 %, −76.2 versus −24.2 %). Drug-related adverse events (AEs) and discontinuations due to drug-related AEs occurred in 15 (14.0 %) versus 8 (7.4 %) patients and 3 (2.8 %) versus 0 patients in the ALN/D5600 and calcitriol group, respectively. Hypercalciuria 12-month incidence (24-h urine Ca >300 mg) was 8.4 (ALN/D5600) versus 13.9 % (calcitriol) (p > 0.05). One patient (calcitriol) had hypercalcemia.
Conclusions
ALN/D5600 produced greater increases in lumbar spine BMD and greater decreases in bone turnover markers versus calcitriol in osteoporotic Chinese women. It is not known whether the greater increase in BMD results in fewer fractures. ALN/D5600 was generally well tolerated in Chinese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is an important public health problem in China, with increased life expectancy and lifestyle changes set to contribute to increased incidence in the future [1]. In 2010, according to the National Bureau of Statistics of China, >169 million (26.0 %) Chinese women were aged over 50 years [2]. The prevalence of osteoporosis in Chinese women aged over 50 years has been estimated at 40.1 % [3]. Prevention and treatment of osteoporosis include ensuring that patients receive adequate calcium and vitamin D [4]. Relatively few types of food contain significant amounts of vitamin D, and as a result, supplements are generally used to treat and prevent vitamin D insufficiency. It has been found that vitamin D insufficiency is a common but largely ignored health problem in China [5, 6], with insufficiency (defined in this case as 25-hydroxyvitamin D (25(OH)D) <20 ng/mL) found in as many as 70–90 % of elderly people in Beijing and Shanghai [6]. A recent large, cross-sectional study conducted in individuals living in northwest China found that the prevalence of 25(OH)D levels <20 ng/mL in the general population of women aged 40–75 years was 80 %, although reduced vitamin D levels were not associated with an increased risk of osteoporosis [7].
The bisphosphonate alendronate has been approved for the treatment of osteoporosis in postmenopausal women at a dose of 10 mg daily or 70 mg once weekly. Alendronate has been shown to increase bone mineral density (BMD) and reduce bone turnover in postmenopausal Chinese women with osteoporosis [8]. A combined formulation of alendronate 70 mg/vitamin D3 5600 IU (ALN/D5600), developed to ensure that osteoporotic patients taking alendronate also receive adequate vitamin D, has also been approved in China.
In this study, the efficacy and safety of ALN/D5600 versus those of calcitriol (1,25(OH)2D3) were compared in Chinese postmenopausal women. Calcitriol, the active hormonal metabolite of vitamin D3, is also currently approved and commonly prescribed for postmenopausal osteoporosis in China. The originally approved dose of calcitriol for postmenopausal osteoporosis was 0.25 μg twice daily; however, due to safety concerns, calcitriol 0.25 μg daily has become the most commonly prescribed regimen in local practice.
We hypothesized that ALN/D5600 administered once weekly as a combination tablet would increase lumbar spine BMD more than calcitriol 0.25 μg daily after both 6 and 12 months treatment. Administration of high doses of either vitamin D3 or calcitriol may produce hypercalcemia and/or hypercalciuria, so both urine and serum calcium levels were monitored as safety end points.
Methods
Study design
This was a 6-month, randomized, open-label, active-comparator-controlled, parallel-group study with a 6-month extension to evaluate the efficacy and safety of ALN/D5600 once weekly as a combination tablet versus those of calcitriol 0.25 μg daily in the treatment of osteoporosis in postmenopausal women in China (protocol number 264–01, clinicaltrials.gov number NCT01350934). After screening, patients were randomized to ALN/D5600 or calcitriol for 6 months in the base study, and for a further 6 months in the extension study. Calcium supplements (500 mg daily) were administered to all patients for the entire 12 months. Study medications were as follows: Fosamax® Plus D (alendronate 70 mg/vitamin D3 5600 IU; manufactured by FROSST IBERICA. SA, a subsidiary of Merck, packaged by MSD, Australia); and Rocaltrol® (calcitriol 0.25 μg; manufactured by R.P. Scherer GmbH & Co. KG under Hoffman-La Roche AG, packaged by Roche, Shanghai, China).
The primary efficacy end point of the base study was percent change from baseline in lumbar spine BMD at month 6. The secondary efficacy end point of the base study was percent change from baseline in the biochemical markers of bone turnover procollagen type 1 N-terminal propeptide (P1NP) and serum C-telopeptides of type 1 collagen (s-CTx) at month 6. Exploratory end points of the base study were at month 6: percent change from baseline in total hip, femoral neck, and trochanter BMD; the proportion of patients with serum 25(OH)D <20 ng/mL; the proportion of patients with serum 25(OH)D <15 ng/mL; the change from baseline in mean serum 25(OH)D level. The safety and tolerability of ALN/D5600 once weekly in this population were also investigated throughout the study.
Efficacy end points of the extension study were at month 12: percent change from baseline in lumbar spine BMD; percent change from baseline in total hip, femoral neck, and trochanter BMD; percent change from baseline in the biochemical markers of bone turnover P1NP and s-CTx; the proportion of patients with serum 25(OH)D <20 ng/mL; the proportion of patients with serum 25(OH)D <15 ng/mL; the change from baseline in mean serum 25(OH)D level.
The study was approved by the independent ethics committees of 13 participating sites. Written informed consent was obtained in accordance with the principles and provisions of the International Conference on Harmonization-E6 Guideline for Good Clinical Practice (GCP) following country-specific consent requirements.
The study was sponsored by MSD China Holding Co., Ltd., China, and jointly administered with PAREXEL China.
Patients
Eligible patients were >55 years of age and postmenopausal for at least 1 year (women who were not postmenopausal were not eligible for inclusion). The patients were also required to have the following: either BMD T-score ≤ -2.5 in at least one anatomic site (lumbar spine, total hip, or femoral neck) or prior non-pathological fragility fracture (of the spine, wrist, humerus, or clavicle) and BMD T-score ≤ -1.5 in at least one of the same anatomic sites; 25(OH)D levels ≥8 ng/mL (20 nmol/L); no prior hip fracture; no abnormal finding of clinical significance (no pronounced medical history other than osteoporosis, including as follows: history of malignancy <5 years prior to signing study consent, with melanoma, leukemia, lymphoma, or myeloproliferative disorders of any duration leading to exclusion; uncontrolled primary/secondary hyperparathyroidism; malabsorption syndrome; history or evidence of metabolic bone disease other than osteoporosis or abnormal serum calcium or phosphate); no treatment with specific drugs for specified time periods (including past oral bisphosphonate or recombinant parathyroid hormone); and no contraindications to alendronate, calcitriol, or vitamin D.
Efficacy assessments
BMD was assessed at screening, month 6 and month 12 via dual-energy X-ray absorptiometry (DXA), using a Hologic (Hologic Corporation) or GE Lunar (GE Medical Systems) densitometer at study sites. The percent change from baseline in lumbar spine, total hip, femoral neck, and trochanter BMD was calculated. Lateral spine X-ray of T4-L5 (one thoracic and one lumbar X-ray) was performed at screening, month 6 and month 12. Biochemical markers of bone turnover, P1NP, and s-CTx were measured at day 1, month 6, and month 12 using P1NP immunoassay and s-CTx electrochemiluminescent immunoassay (Roche Cobas E601 platform using Roche reagents), and assessed at a central laboratory (Quest Diagnostics, Shanghai, China). A constrained longitudinal data analysis (cLDA) method was used to analyze these biochemical markers.
Serum 25(OH)D levels were assessed using liquid chromatography-tandem mass spectrometry (LCMSMS; API4000 platform) at a central laboratory (Quest Diagnostics, Shanghai, China) and monitored throughout the study. The total of both 25(OH)D3 and 25(OH)D2 was reported as 25(OH)D.
Safety assessments
Safety was assessed using reported adverse events (AEs) and laboratory safety evaluations (including the following: blood chemistry and urinalysis, with but not limited to, evaluation of albumin, blood urea nitrogen, and creatinine; serum calcium; and 24-h urine calcium), physical examinations, measurement of vital signs, and 12-lead electrocardiogram (ECG). Hypercalcemia (defined as total serum calcium (not corrected for protein) >2.60 mmol/L) and hypercalciuria (defined as 24-h urine calcium excretion >300 mg) were safety events of special interest. Serum calcium was assessed at screening, randomization, and at 3, 6, 9, and 12 months, and 24-h urinary calcium was assessed at randomization and at 6 and 12 months.
Statistical analyses
The base study planned to enroll 100 patients per group in a 1:1 ratio to receive ALN/D5600 or calcitriol. The study had approximately 97 % power (2-sided, α = 0.05) to demonstrate the primary hypothesis that treatment with ALN/D5600 was superior to treatment with calcitriol as measured by percent change from baseline in lumbar spine BMD at month 6. This was based on the following assumptions: (a) an underlying 2 % treatment difference between ALN/D5600 IU and calcitriol, (b) a standard deviation (SD) of 3.3 % for percent change from baseline in lumbar spine BMD at month 6, and (c) approximately 15 % of the patients would discontinue prior to month 6 (for any reason). The extension study had more than 99 % power (2-sided, α = 0.05) to demonstrate that treatment with ALN/D5600 IU was superior to treatment with calcitriol as measured by percent change from baseline in the lumbar spine BMD at month 12 based on the following assumptions: (a) an underlying 4 % treatment difference between ALN/D5600 IU and calcitriol, (b) an SD of 3.3 % for percent change from baseline in lumbar spine BMD at month 12, and (c) a further 5 % of patients would discontinue prior to month 12.
Efficacy analyses were performed on the full analysis set (FAS) comprising all randomized patients who were subject to ≥1 dose of study treatment and ≥1 post-randomization observation for analysis end point subsequent to ≥1 dose of study treatment, and the per-protocol set (PPS), which excluded patients due to major protocol deviations. The primary analysis population was the FAS for assessment of changes in BMD and the PPS for bone turnover indices. A longitudinal data analysis method was used to determine percent changes from baseline in each of the efficacy end points, with adjustments for treatment, time, interactions of time by treatment, and baseline covariates such as 25(OH)D level stratum (≤15 versus >15 ng/mL). For biochemical markers of bone turnover, P1NP and s-CTx, percent change from baseline in log scale was the analytic metrics. The estimates of the between- and within-group treatment means and the associated 95 % confidence intervals (CIs) were back-transformed for presentation.
Safety data were analyzed in the all patients as treated (APaT) population (all randomized patients who received ≥1 dose of study treatment). The analysis of safety results followed a tiered approach. The proportion of patients who developed either hypercalcemia or hypercalciuria was identified a priori as an AE of special interest and was considered a tier 1 event. AEs and predefined limits of change in laboratory values, vital signs, and ECG parameters were classified as tier 2 or tier 3 events based on the number of events observed. P values (tier 1 only) and 95 % CIs (tier 1 and tier 2) for between-treatment differences in the percent of patients with events were calculated using the Miettinen and Nurminen method [9].
Results
Patients
From July 2011 through January 2013, 304 patients were screened from 13 centers in China, of whom 219 (72.0 %) were randomized to receive either ALN/D5600 (n = 111) or calcitriol (n = 108) (Fig. 1). Four patients (1.8 %) from the ALN/D5600 group were randomized but did not receive the study drug. A total of 196 (89.5 %) patients completed the study, and 23 (10.5 %) patients discontinued from the study. The most common reason for early discontinuation was an AE, which was reported in 8 (7.2 %) patients from the ALN/D5600 group and 1 (0.9 %) patient from the calcitriol group.
Demographic and baseline characteristics were comparable between treatment groups (Table 1). Overall, the mean age was 65 years and all patients were Asian, except two in the calcitriol group who were Caucasian. Mean 25(OH)D levels were 19.8 and 19.4 ng/mL in the ALN/D5600 and calcitriol groups, respectively, and the percentages of patients with 25(OH)D values of ≤15 and >15 ng/mL were similar in both treatment groups. Of note, 63/208 (30.3 %) patients had a 25(OH)D level of ≤15 ng/mL. Use of either calcium carbonate plus cholecalciferol or other unspecified vitamin D forms was reported by 4.3 and 3.8 % of patients, respectively.
Efficacy
Lumbar spine, total hip, femoral neck, and trochanter BMD
Percent changes from baseline (least squares (LS) mean) of the lumbar spine BMD at month 6 (primary efficacy end point) in the FAS were 3.5 % (95 % CI 2.7, 4.4; standard error (SE), 0.42) and 1.6 % (95 % CI 0.8, 2.4; SE 0.41) for ALN/D5600 and calcitriol, respectively; there was a between-treatment difference of 2.0 % (95 % CI 0.8, 3.1; p < 0.001 adjusted for 25(OH)D level stratum as a baseline covariate) (Fig. 2a). At month 12, the percent changes (LS mean) of lumbar spine BMD were 5.2 % (95 % CI 4.3, 6.0) and 2.3 % (95 % CI 1.4, 3.1) for the ALN/D5600 and calcitriol groups, respectively,; there was a between-treatment difference of 2.9 % (95 % CI 1.8, 4.1; p < 0.001 adjusted for 25(OH)D level stratum as a baseline covariate). Analysis in the PPS suggested a similar trend in terms of increase in the lumbar spine BMD at months 6 and 12, supporting the primary analysis result.
Within-treatment differences in BMD percent changes from baseline (LS mean) of the total hip, femoral neck, and trochanter at month 6 for ALN/D5600 were 1.6 % (95 % CI 0.9, 2.4), 2.8 % (95 % CI 1.2, 4.3), and 2.2 % (95 % CI 0.9, 3.5), and for calcitriol were 0.5 % (95 % CI −0.2, 1.2), 0.8 % (95 % CI −0.7, 2.2), and 0.4 % (95 % CI −0.8, 1.7), respectively (Fig. 2b–d). Corresponding data at month 12 for ALN/D5600 were 2.7 % (95 % CI 1.7, 3.6), 3.3 % (95 % CI 1.9, 4.7), and 2.4 % (95 % CI 0.9, 3.8), and for calcitriol were 0.1 % (95 % CI −0.8, 1.0), 0.4 % (95 % CI −0.9, 1.8), and 0.1 % (95 % CI −1.3, 1.5), respectively. Between-treatment differences were statistically significant for the total hip and trochanter at month 6, and for the total hip, femoral neck, and trochanter at month 12.
Bone turnover biomarkers
At months 6 and 12, percent changes from baseline in P1NP (Fig. 3a) and s-CTx (Fig. 3b) (determined for the PPS) showed significantly greater decreases with ALN/D5600 than with calcitriol.
Percent change from baseline in bone turnover markers: a P1NP and b s-CTx (FAS). ALN/D5600 combination tablet of alendronate 70 mg plus vitamin D3 5600 IU once weekly, CI confidence interval, FAS full analysis set, LS least squares, P1NP procollagen of type 1 N-terminal propeptide, s-CTx C-telopeptides of type 1 collagen
Within-treatment differences in percent changes from baseline (LS mean) of P1NP and s-CTx at month 6 for ALN/D5600 were −59.1 % (95 % CI −62.0, −56.0) and −79.2 % (95 % CI −81.4, −76.8), and for calcitriol were −16.8 % (95 % CI −22.5, −10.5) and −27.2 % (95 % CI −34.6, −19.0), respectively. Corresponding data at month 12 for ALN/D5600 were −68.1 % (95 % CI −70.8, −65.1) and −76.2 % (95 % CI −78.6, −73.5), and for calcitriol were −17.0 % (95 % CI −23.8, −9.6) and −24.2 % (95 % CI −31.6, −16.0), respectively. Between-treatment differences were statistically significant for P1NP and s-CTx at months 6 and 12.
Serum 25(OH)D
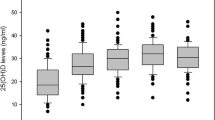
Figure 4 shows (a) changes from baseline in 25(OH)D, and the distribution of 25(OH)D concentrations after treatment with ALN/D5600 or calcitriol at month 6 (b) and month 12 (c) in the FAS. Mean 25(OH)D levels in the ALN/D5600 group increased from 19.7 ng/mL at baseline to 29.4 and 30.2 ng/mL at months 6 and 12, respectively. In the calcitriol group, mean 25(OH)D levels decreased slightly from baseline (19.4 ng/mL) to month 6 (16.1 ng/mL) but were similar to baseline levels at month 12 (20.3 ng/mL). At month 6, the proportion of patients with 25(OH)D defined thresholds (<15 and <20 ng/mL) was significantly higher in the calcitriol group (48.6 and 72.9 %, respectively) than in the ALN/D5600 group (1.0 and 5.9 %, respectively) (both, p < 0.001). By month 12, the proportion of patients with 25(OH)D below the same thresholds (<15 and <20 ng/mL) had decreased in the calcitriol group (25.5 and 47.1 %, respectively) and remained very low in the ALN/D5600 group (1.0 and 4.1 %, respectively) (both, p < 0.001). Similar results were observed in the PPS.
Changes from baseline in serum 25(OH)D concentrations after treatment with ALN/D5600 or calcitriol (FAS). ALN/D5600 combination tablet of alendronate 70 mg plus vitamin D3 5600 IU once weekly, FAS full analysis set, SE standard error, 25(OH)D 25-hydroxy vitamin D. *Post hoc analysis: two-sample independent t test without adjustments for covariates
Safety and tolerability
Table 2 presents all AEs with an incidence >5 % in either treatment group. All randomized patients who were administered at least one dose of the study drug (N = 215) were included in the evaluation of safety (APaT population). The overall safety profile appeared to be similar between groups. While no statistical testing was conducted, small differences in AE incidence were generally consistent with the recognized safety profile of each treatment.
By the end of month 12, the numbers and proportion of patients with at least one AE were 64/107 (59.8 %) and 74/108 (68.5 %) in the ALN/D5600 and calcitriol groups, respectively. The number of patients with drug-related AEs was higher in the ALN/D5600 group (14.0 %) than that in the calcitriol group (7.4 %). The most frequently reported drug-related AE in the ALN/D5600 group was upper abdominal pain (3.7 %). The proportion of patients with at least one serious AE (SAE) in the ALN/D5600 group (3.7 %) and in the calcitriol group (4.6 %) was similar. None of the SAEs were considered to be related to the study drug. Discontinuations due to AEs occurred in 7 (6.5 %) patients in the ALN/D5600 group and 3 (2.8 %) patients in the calcitriol group; these were assessed as drug related in 3 (2.8 %; gastrointestinal disorders including abdominal discomfort, gastritis, and esophageal pain which were reported by 1 patient each) and 0 patients, respectively. No deaths were reported. Fractures were reported by 1 patient (0.9 %: patella fracture, SAE) in the ALN/D5600 group and by 4 patients (3.7 %: 2 patients with femoral neck fractures, both considered an SAE; 1 patient with a non-serious spinal compression fracture and a non-serious foot fracture; 1 patient with a spinal compression and a vertebral fracture, both considered an SAE) in the calcitriol group.
Differences in the incidence of hypercalcemia, hypercalciuria, or either of these two events between ALN/D5600 and calcitriol groups at month 12 were not statistically significant (Table 2, post hoc analysis). There was 1/108 (0.9 %) patient in the calcitriol group who was defined as having hypercalcemia (serum non-corrected calcium >2.60 mmol/L) during the study.
No clinically important findings were observed in laboratory values and vital signs, with no notable differences between the two treatment groups. There was a higher decrease in total alkaline phosphatase over time with ALN/D5600 than with calcitriol (decreases of -22.1 and -6.5 U/L, respectively, by month 12). No laboratory abnormalities were reported as drug-related SAEs or drug-related AEs that led to study drug discontinuation.
Discussion
This study investigated the efficacy and safety of ALN/D5600 once weekly compared with those of calcitriol in Chinese postmenopausal women. To our knowledge, this was the first clinical study in China to compare the therapeutic profiles of a fixed-dose combination of a bisphosphonate and vitamin D3 directly with calcitriol treatment in the clinical setting. In the study, 215 patients received at least one dose of the study drug and 89.5 % (196/219) of patients completed the 6-month extension period, indicating a good patient retention. Consistent with the stated hypothesis and the primary efficacy end point of the study, ALN/D5600 was superior to calcitriol as measured by percent change from baseline in lumbar spine BMD at month 6, with a treatment difference of 2.0 % (p < 0.001, adjusted for baseline 25(OH)D level) in favor of ALN/D5600. This difference was maintained at month 12, with a treatment difference of 2.9 % (p < 0.001). ALN/D5600 also increased BMD at the total hip, the femoral neck, and the trochanter more than calcitriol 0.25 μg daily after 12 months of treatment. The within-group increases in BMD at both month 6 and month 12 in the ALN/D5600 group are consistent with the results of previous studies using alendronate 70 mg once weekly or 10 mg daily doses of alendronate [10–16]. Treatment with ALN/D5600 also resulted in significantly greater reductions from baseline compared with that with calcitriol in the bone turnover markers, P1NP and s-CTx, at both month 6 and month 12.
Although fracture risk reduction is the ultimate goal of osteoporosis therapy, the present study was not sufficiently powered to detect a statistically meaningful difference in either vertebral or non-vertebral clinical fracture incidence between the two treatment groups. Rather, the study’s aim was to explore BMD changes and its related changes in biochemical markers for the assessment of efficacy. In the entire study, fractures were detected in 5 patients, 4 (3.7 %) in the calcitriol group (2 [1.9 %] with vertebral fractures and 2 [1.9 %] with femoral neck fractures), and 1 (0.9 %) in the ALN/D5600 group (patella fracture). Previous large studies have shown that treatment with alendronate for 3 or 4 years significantly increases BMD and reduces the risk of morphometric vertebral fractures in women with low BMD but no vertebral fractures [17] and both hip and spine fractures in women with low BMD and previous vertebral fracture [18]. In a subgroup of patients without prior vertebral fractures with hip neck BMD T-scores < –2.5, the risk of vertebral, hip, and a composite end point of any clinical fracture were reduced in patients treated with alendronate [17]. It is therefore reasonable to postulate that treatment with ALN/D5600 would have a future clinical benefit in reducing vertebral and non-vertebral fracture incidence in Chinese postmenopausal women with osteoporosis. There are no fracture studies on calcitriol 0.25 μg daily for comparison; however, a 3-year study of 622 women with postmenopausal osteoporosis and one or more vertebral compression fractures showed that calcitriol 0.25 μg twice daily significantly reduced the incidence of new vertebral fractures compared with calcium [19].
In patients with osteoporosis, low 25(OH)D levels are prevalent, regardless of geographic location [20]. In China and most other countries, vitamin D supplementation, preferably 800 IU daily or above, and calcium are recommended for use along with anti-resorptive therapies based on current guidelines [4, 21, 22]. In this study population, the median 25(OH)D level at baseline was 18.5 ng/mL, which indicates that more than 50 % of patients had a vitamin D level <20 ng/mL. In addition, 30.3 % of patients had a vitamin D level <15 ng/mL. As in previous studies [23, 24], ALN/D5600 effectively increased 25(OH)D levels: mean 25(OH)D increased from 19.7 ng/mL at baseline to 29.4 ng/mL and 30.2 ng/mL at months 6 and 12, respectively, and the proportion of patients whose vitamin D levels were <15 and <20 ng/mL was 1.0 and 5.9 %, respectively, at month 6, and 1.0 and 4.1 %, respectively, at month 12. Based on these data, ALN/D5600 is a rational combination treatment option to help ensure that patients receiving alendronate in China also have adequate vitamin D.
In the calcitriol group, a decrease in mean 25(OH)D level was observed at month 6 (from 19.4 to 16.1 ng/mL) with a return to baseline (to 20.3 ng/mL) at month 12. One reason for the initial decline might be that 1,25(OH)2D stimulates 25(OH)D catabolism. The body responds to 1,25(OH)2D by enhancing expression of 25(OH)D-24-hydroxylase (CYP24A1) to metabolize 25(OH)D and 1,25(OH)2D into water-soluble inactive forms that are excreted in the bile [25]. Additionally, efficient patient enrollment was seen in the fall 2011, with the first 6 months of the study including the winter period when lack of sun exposure could have led to decreased 25(OH)D levels. Low 25(OH)D levels may not, however, be important in patients who are being administered calcitriol directly (taking into consideration calcitriol’s direct effect on calcium absorption resulting in an effect on bone turnover and lowering of PTH).
ALN/D5600 was generally well tolerated, and the safety profile was consistent with that previously reported [23, 24] and with the current product package insert. The incidence of drug-related AEs was almost twice as high in the ALN/D5600 group compared with that in the calcitriol group (14.0 versus 7.4 %), with upper abdominal pain as the most common drug-related AE reported among ALN/D5600-treated patients (3.7 %). Discontinuations due to drug-related AEs were also more common in the ALN/D5600 group (2.8 versus 0 %) and were due to gastrointestinal disorders. This is consistent with placebo-controlled trials of oral alendronate. Although inference testing did not show statistical significance (p not less than 0.05), there was a slightly higher incidence of hypercalciuria (events reported either as “hypercalciuria” or “urine calcium increased”) with calcitriol than with ALN/D5600 (13.9 versus 8.4 % at month 12). In addition, there was only one event of hypercalcemia reported, which was in the calcitriol group.
A limitation of this study was the open-label design which may have introduced bias. In addition, although BMD was measured by either Hologic (Hologic Corporation) or GE Lunar (GE Medical Systems) DXA machines and individual sites followed quality control procedures recommended by the manufacturers, there was no independent central BMD quality control/quality assessment program. However, because the study was not large enough to compare fracture incidence, central adjudication of events such as fractures was not performed by either an internal or an external committee. Moreover, the study was not designed to distinguish the relative contributions of ALN and vitamin D3 in reducing bone loss and increasing BMD. In this study, parathyroid hormone (PTH) levels were not measured over time, and thus, the interaction between vitamin D and the anti-resorptive effect of ALN could not be interpreted fully. A previous study showed that while administration of vitamin D was not a requirement for ALN to decrease bone turnover in patients with sufficient vitamin D, it did result in approximately 25 % greater fall in s-CTx in patients with vitamin D insufficiency (defined as 25(OH)D <20 ng/mL) [26]. The small within-group decreases in bone turnover markers in the calcitriol group could have been due to the start of either calcium supplements or calcitriol (or the net effect of both). Other studies assessing the effects of 25(OH)D levels on BMD changes with ALN treatment indicate that there is a minimum required 25(OH)D level for an optimal effect of ALN. While BMD response may not vary with baseline vitamin D levels when patients are supplemented with vitamin D3, BMD response is correlated with 25(OH)D levels achieved by the end of treatment with combined ALN and vitamin D [27–29].
In summary, ALN/D5600 showed significantly greater increases in BMD at the lumbar spine and significantly greater decreases in bone turnover markers compared with calcitriol in Chinese postmenopausal women with osteoporosis. The study did not evaluate whether the greater increase in BMD resulted in fewer fractures. ALN/D5600 was also generally well tolerated in Chinese patients with a safety profile consistent with previous studies across various regions and races.
References
International Osteoporosis Foundation (2013) The Asia-Pacific regional audit. Epidemiology, costs & burden of osteoporosis in 2013. http://www.iofbonehealth.org/data-publications/regional-audits/asia-pacific-regional-audit. Accessed 31 March 2015
National Bureau of Statistics of China (2010) 2010 Population Census of the People’s Republic of China. http://www.stats.gov.cn/english./Statisticaldata/CensusData. Accessed 31 March 2015
Wang Y, Tao Y, Hyman ME, Li J, Chen Y (2009) Osteoporosis in China. Osteoporos Int 20:1651–1662
National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington
Wat WZ, Leung JY, Tam S, Kung AW (2007) Prevalence and impact of vitamin D insufficiency in southern Chinese adults. Ann Nutr Metab 51:59–64
Zhang W, Stoecklin E, Eggersdorfer M (2013) A glimpse of vitamin D status in Mainland China. Nutrition 29:953–957
Zhen D, Liu L, Guan C, Zhao N, Tang X (2015) High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone 71:1–6
Yan Y, Wang W, Zhu H, Li M, Liu J, Luo B, Xie H, Zhang G, Li F (2009) The efficacy and tolerability of once-weekly alendronate 70 mg on bone mineral density and bone turnover markers in postmenopausal Chinese women with osteoporosis. J Bone Miner Metab 27:471–478
Miettinen O, Nurminen M (1985) Comparative analysis of two rates. Stat Med 4:213–226
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 9:461–468
Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, Felsenberg D, Recker RR, Tonino RP, Roux C, Pinchera A, Foldes AJ, Greenspan SL, Levine MA, Emkey R, Santora AC, Kaur A, Thompson DE, Yates J, Orloff JJ (2000) Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 12:1–12
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Weryha G, Marques-Neto JF, Gaines KA, Verbruggen N, Melton ME (2006) Alendronic acid produces greater effects than risedronic acid on bone density and turnover in postmenopausal women with osteoporosis: results of FACTS-international. Clin Drug Investig 26:63–74
Sambrook PN, Geusens P, Ribot C, Solimano JA, Ferrer-Barriendos J, Gaines K, Verbruggen N, Melton ME (2004) Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: results of EFFECT (Efficacy of FOSAMAX versus EVISTA Comparison Trial) International. J Intern Med 255:503–511
Luckey M, Kagan R, Greenspan S, Bone H, Kiel RD, Simon J, Sackarowitz J, Palmisano J, Chen E, Petruschke RA, de Papp AE (2004) Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause 11:405–415
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Tilyard MW, Spears GF, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326:357–362
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Society of Osteoporosis and Bone Mineral Research Chinese Medical Association (2011) Guidelines for diagnosis and treatment of primary osteoporosis. Chin J Osteoporos Bone Miner Res 4:2–17
Rizzoli R, Boonen S, Brandi ML, Bruyere O, Cooper C, Kanis JA, Kaufman JM, Ringe JD, Weryha G (2013) Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 29:305–313
Binkley N, Ringe JD, Reed JI, Ljunggren O, Holick MF, Minne HW, Liu M, Lamotta A, West JA, Santora AC (2009) Alendronate/vitamin D3 70 mg/2800 IU with and without additional 2800 IU vitamin D3 for osteoporosis: results from the 24-week extension of a 15-week randomized, controlled trial. Bone 44:639–647
Ralston SH, Binkley N, Boonen S, Kiel DP, Reginster JY, Roux C, Chen L, Rosenberg E, Santora A (2011) Randomized trial of alendronate plus vitamin D3 versus standard care in osteoporotic postmenopausal women with vitamin D insufficiency. Calcif Tissue Int 88:485–494
Hossein-nezhad A, Holick MF (2013) Vitamin D for health: a global perspective. Mayo Clin Proc 88:720–755
Olmos JM, Hernandez JL, Llorca J, Nan D, Valero C, Gonzalez-Macias J (2012) Effects of 25-hydroxyvitamin D3 therapy on bone turnover markers and PTH levels in postmenopausal osteoporotic women treated with alendronate. J Clin Endocrinol Metab 97:4491–4497
Ishijima M, Sakamoto Y, Yamanaka M, Tokita A, Kitahara K, Kaneko H, Kurosawa H (2009) Minimum required vitamin D level for optimal increase in bone mineral density with alendronate treatment in osteoporotic women. Calcif Tissue Int 85:398–404
Antoniucci DM, Vittinghoff E, Palermo L, Black DM, Sellmeyer DE (2009) Vitamin D insufficiency does not affect response of bone mineral density to alendronate. Osteoporos Int 20:1259–1266
Roux C, Binkley N, Boonen S, Kiel DP, Ralston SH, Regnister JY, Pong A, Rosenberg E, Santora A (2014) Vitamin D status and bone mineral density changes during alendronate treatment in postmenopausal osteoporosis. Calcif Tissue Int 94:153–157
Acknowledgments
This study was sponsored by MSD China Holding Co., Ltd., China. We thank Annette Smith, PhD, who provided the medical writing support on behalf of the Complete Medical Communications, funded by Merck & Co., Inc., Whitehouse Station, NJ, USA. Further medical writing assistance was provided by Elizabeth Rosenberg, formerly of Merck & Co., Inc.
Conflicts of interest
Fang Wei, Jue Wang, Lei Zhang (MSD China Holding Co., Ltd), and Arthur C Santora (Merck Research Laboratories, Rahway, NJ, USA) are or were, at the time of study conduct, employees of the study sponsor (Merck).
Fang Wei, Lei Zhang, and Arthur C Santora own a stock in Merck.
Zhen Lin Zhang, Er Yuan Liao, Wei Bo Xia, Hua Lin, and De Cai Chen have received research support from Merck.
Zhen Lin Zhang, Er Yuan Liao, Wei Bo Xia, Hua Lin, De Cai Chen, and Hai Tang have been consultants for Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, Z.L., Liao, E.Y., Xia, W.B. et al. Alendronate sodium/vitamin D3 combination tablet versus calcitriol for osteoporosis in Chinese postmenopausal women: a 6-month, randomized, open-label, active-comparator-controlled study with a 6-month extension. Osteoporos Int 26, 2365–2374 (2015). https://doi.org/10.1007/s00198-015-3141-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3141-y