Abstract
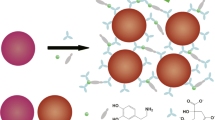
A colorimetric/fluorometric dual-signal assay is described for the determination of dopamine (DA). A nanoprobe was obtained by linking a 4-(4-dialkylaminostyryl)pyridinium derived fluorophore to citrate-capped gold nanoparticles (AuNPs). The fluorescence of the fluorophore is quenched by the AuNPs via fluorescence resonance energy transfe. In the presence of DA, the catechol group of DA can absorb on the surface of AuNPs to induce aggregation, which is accompanied by a color change from red to blue. The yellow fluorescence of the fluorophore with excitation/emission maximum at 365/570 nm is recovered. The dual-signal detection allows the quantitative analysis of DA within 300 μM by the colorimetric method and 80 μM by the fluorometric method. The detection limits for the colorimetric/fluorometric methods are 1.85 μM and 0.29 μM, respectively. Quantitative determination of DA in spiked urine samples was successfully demonstrated, with recoveries ranging from 98.2 to 106.0%.

A colorimetric/fluorometric dual-signal assay is described for the determination of dopamine by linking a fluorophore to gold nanoparticles. The dopamine causes aggregation of the nanoparticles to induce color change, which is followed by the recovery of the fluorescence.







Similar content being viewed by others
References
Rodriguez PC, Pereira DB, Borgkvist A, Wong MY, Barnard C, Sonders MS, Zhang H, Sames D, Sulzer D (2013) Fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. P Natl Acad Sci USA 110:870–875
Gingrich JA, Caron MG (1993) Recent advances in the molecular biology of dopamine receptors. Annu Rev Neuro Sci 16:299–321
Ali SR, Ma Y, Parajuli RR, Balogun Y, Lai WYC, He H (2007) A nonoxidative sensor based on a self-doped polyaniline/carbon nanotube composite for sensitive and selective detection of the neurotransmitter dopamine. Anal Chem 79:2583–2587
Merims D, Giladi N (2008) Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease. Parkinsonism Relat D 14:273–280
Tsai T-C, Huang F-H, Chen J-JJ (2013) Selective detection of dopamine in urine with electrodes modified by gold nanodendrite and anionic self-assembled monolayer. Sensor Actuat B: Chem 181:179–186
Fischbach FT (2000) A manual of laboratory and diagnostic tests (6 ed.) Lippincott Williams & Wilkins
Chiara GD (1990) In-vivo brain dialysis of neurotransmitters. Trends in Pharmacol Sci 11:116–121
Park YH, Zhang X, Rubakhin SS, Sweedler JV (1999) Independent optimization of capillary electrophoresis separation and native fluorescence detection conditions for indolamine and catecholamine measurements. Anal Chem 71:4997–5002
Syslová K, Rambousek L, Kuzma M, Najmanová V, Bubeníková-Valešová V, Šlamberová R, Kačer P (2011) Monitoring of dopamine and its metabolites in brain microdialysates: method combining freeze-drying with liquid chromatography–tandem mass spectrometry. J Chromatogr A 1218:3382–3391
Lee MH, Kim JS, Sessler JL (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 44:4185–4191
Wu J, Liu W, Ge J, Zhang H, Wang P (2011) New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 40:3483–3495
Xu J, Yu H, Hu Y, Chen M, Shao S (2016) A gold nanoparticle-based fluorescence sensor for high sensitive and selective detection of thiols in living cells. Biosens Bioelectron 75:1–7
Rawat KA, Bhamore JR, Singhal RK, Kailasa SK (2017) Microwave assisted synthesis of tyrosine protected gold nanoparticles for dual (colorimetric and fluorimetric) detection of spermine and spermidine in biological samples. Biosens Bioelectron 88:71–77
Li N, Zhao P, Astruc D (2014) Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew Chem Inter Ed 53:1756–1789
Ahmad R, Griffete N, Lamouri A, Felidj N, Chehimi MM, Mangeney C (2015) Nanocomposites of gold nanoparticles@molecularly imprinted polymers: chemistry, processing, and applications in sensors. Chem Mater 27:5464–5478
Ju J, Chen W (2015) In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal Chem 87:1903–1910
Sener G, Uzun L, Denizli A (2014) Lysine-promoted colorimetric response of gold nanoparticles: a simple assay for ultrasensitive mercury(II) detection. Anal Chem 86:514–520
Cheng Y-H, Tang H, Jiang J-H (2017) Enzyme mediated assembly of gold nanoparticles for ultrasensitive colorimetric detection of hepatitis C virus antibody. Anal Methods 9:3777–3781
Yu XF, Wang DS, Peng Q, Li YD (2011) High performance electrocatalyst: Pt-Cu hollow nanocrystals. Chem Commun 47:8094–8096
Yu M, Wang H, Fu F, Li L, Li J, Li G, Song Y, Swihart MT, Song E (2017) Dual-recognition Förster resonance energy transfer based platform for one-step sensitive detection of pathogenic bacteria using fluorescent vancomycin–gold nanoclusters and aptamer–gold nanoparticles. Anal Chem 89:4085–4090
Zheng L, Wei J, Lv X, Bi Y, Wu P, Zhang Z, Wang P, Liu R, Jiang J, Cong H, Liang J, Chen W, Cao H, Liu W, Gao GF, Du Y, Jiang X, Li X (2017) Detection and differentiation of influenza viruses with glycan-functionalized gold nanoparticles. Biosens Bioelectron 91:46–52
Rivero PJ, Ibañez E, Goicoechea J, Urrutia A, Matias IR, Arregui FJ (2017) A self-referenced optical colorimetric sensor based on sil ver and gold nanoparticles for quantitative determination of hydrogen peroxide. Sensor Actuat B: Chem 251:624–631
Shahar T, Sicron T, Mandler D (2017) Nanosphere molecularly imprinted polymers doped with gold nanoparticles for high selectivity molecular sensors. Nano Res 10:1056–1063
Ben Messaoud N, Ghica ME, Dridi C, Ben Ali M, Brett CMA (2017) Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sensor Actuat B: Chem 253:513–522
Kanayama N, Takarada T, Maeda M (2011) Rapid naked-eye detection of mercury ions based on non-crosslinking aggregation of double-stranded DNA-carrying gold nanoparticles. Chem Commun 47:2077–2079
Cao R, Li B, Zhang Y, Zhang Z (2011) Naked-eye sensitive detection of nuclease activity using positively-charged gold nanoparticles as colorimetric probes. Chem Commun 47:12301–12303
Chen S-J, Chang H-T (2004) Nile red-adsorbed gold nanoparticles for selective determination of thiols based on energy transfer and aggregation. Anal Chem 76:3727–3734
Lo S-H, Wu M-C, Wu S-P (2015) A turn-on fluorescent sensor for cysteine based on BODIPY functionalized Au nanoparticles and its application in living cell imaging. Sensor Actuat B: Chem 221:366–1371
He X, Liu H, Li Y, Wang S, Li Y, Wang N, Xiao J, Xu X, Zhu D (2005) Gold nanoparticle-based fluorometric and colorimetric sensing of copper(II) ions. Adv Mater 17:2811–2815
Ipe BI, Yoosaf K, Thomas KG (2006) Functionalized gold nanoparticles as phosphorescent nanomaterials and sensors. J Am Chem Soc 128:1907–1913
Huang C-C, Chang H-T (2006) Selective gold-nanoparticle-based "turn-on" fluorescent sensors for detection of mercury(II) in aqueous solution. Anal Chem 78:8332–8338
Zhang N, Liu Y, Tong L, Xu K, Zhuo L, Tang B (2008) A novel assembly of Au NPs–β-CDs–FL for the fluorescent probing of cholesterol and its application in blood serum. Analyst 133:1176–1181
Lin Y, Chen C, Wang C, Pu F, Ren J, Qu X (2011) Silver nanoprobe for sensitive and selective colorimetric detection of dopaminevia robust Ag-catechol interaction. Chem Commun 47:1181–1183
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Inter Sci 26:62–69
Li JF, Huang YF, Ding Y, Yang ZL, Li SB, Zhou XS, Fan FR, Zhang W, Zhou ZY, Wu DY, Ren B, Wang ZL, Tian ZQ (2010) Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464:392–395
Chen J, Li Y, Huang Y, Zhang H, Chen X, Qiu H (2019) Fluorometric dopamine assay based on an energy transfer system composed of aptamer-functionalized MoS2 quantum dots and MoS2 nanosheets. Microchim Acta 186:58
Acknowledgments
We sincerely acknowledge the financial support of the National Natural Science Foundation of China (81701766 and 81702998).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2.76 mb)
Rights and permissions
About this article
Cite this article
Peng, J., Zhou, N., Zhong, Y. et al. Gold nanoparticle-based detection of dopamine based on fluorescence resonance energy transfer between a 4-(4-dialkylaminostyryl)pyridinium derived fluorophore and citrate-capped gold nanoparticles. Microchim Acta 186, 618 (2019). https://doi.org/10.1007/s00604-019-3727-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3727-8