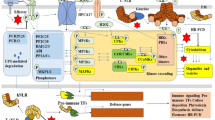
Abstract
Harnessing the toxic properties of reactive oxygen species (ROS) to fight off invading pathogens can be considered a major evolutionary success story. All aerobic organisms have evolved the ability to regulate the levels of these toxic intermediates, whereas some have evolved elaborate signalling pathways to dramatically increase the levels of ROS and use them as weapons in mounting a defence response, a process commonly referred to as the oxidative burst. The balance between steady state levels of ROS and the exponential increase in these levels during the oxidative burst has begun to shed light on complex signalling networks mediated by these molecules. Here, we discuss the different sources of ROS that are present in plant cells and review their role in the oxidative burst. We further describe two well-studied ROS generating systems, the NADPH oxidase and apoplastic peroxidase proteins, and their role as the primary producers of ROS during pathogen invasion. We then discuss what is known about the metabolic and proteomic fluxes that occur in plant cells during the oxidative burst and after pathogen recognition, and try to highlight underlying biochemical processes that may provide more insight on the complex regulation of ROS in plants.



Similar content being viewed by others
References
Albenne CC, Canut H, Boudart G, Zhang Y, San Clemente HLN, Pont-Lezica R, Jamet E (2009) Plant cell wall proteomics: mass spectrometry data, a trove for research on protein structure/function relationships. Mol Plant 2:977–989
Allwood WJ, Clarke A, Goodacre R, Mur LAJ (2010) Dual metabolomics: a novel approach to understanding plant–pathogen interactions. Phytochemistry 71:590–597
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol 90:109–116
Bach M, Schnitzler JP, Seitz HU (1993) Elicitor-induced changes in Ca++ influx, K+ efflux, and 4-hydroxybenzoic acid synthesis in protoplasts of Daucus carota L. Plant Physiol 103:407–412
Badri DV, Quintana N, El Kassis EG, Kim HK, Choi YH, Sugiyama A, Verpoorte R, Martinoia E, Manter DK, Vivanco JM (2009) An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. Plant Physiol 151:2006–2017
Baker C, Roberts D, Mock N (2005) Apoplastic redox metabolism: synergistic phenolic oxidation and a novel oxidative burst. Physiol Mol Plant Pathol 67:296–303
Baker CJ, Orlandi EW (1995) Active oxygen in plant pathogenesis. Annu Rev Phytopathol 33:299–321
Baker CJ, Orlandi EW, Deahl KL (2000) Oxygen metabolism in plant/bacteria interactions: characterization of the oxygen uptake response of plant suspension cells. Physiol Mol Plant Pathol 57:159–167
Barceló RA (1998) The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207:207–216
Bayer EM, Bottrill AR, Walshaw J, Vigouroux M, Naldrett MJ, Thomas CL, Maule AJ (2006) Arabidopsis cell wall proteome defined using multidimensional protein identification technology. Proteomics 6:301–311
Berglund GI, Carlsson GH, Smith AT, Szoke H, Henriksen A, Hajdu J (2002) The catalytic pathway of horseradish peroxidase at high resolution. Nature 417:463–468
Bestwick C, Brown I, Mansfield J (1998) Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol 118:1067–1078
Bindschedler L, Dewdney J, Blee K, Stone J, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies D (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47:851–863
Bindschedler LV, Minibayeva F, Gardner SL, Gerrish C, Davies DR, Bolwell GP (2001) Early signalling events in the apoplastic oxidative burst in suspension cultured French bean cells involve cAMP and Ca2+. New Phytol 151:185–194
Blee KA, Jupe SC, Richard G, Zimmerlin A, Davies DR, Bolwell GP (2001) Molecular identification and expression of the peroxidase responsible for the oxidative burst in French bean (Phaseolus vulgaris L.) and related members of the gene family. Plant Mol Biol 47:607–620
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor-mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12:1425–1440
Bolwell GP (1987) Elicitor induction of the synthesis of a novel lectin-like arabinosylated hydroxyproline-rich glycoprotein in suspension cultures of Phaseolus vulgaris L. Planta 172:184–191
Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence, a broad perspective. Physiol Mol Plant Pathol 51:347–366
Bolwell GP, Daudi A (2009) Reactive oxygen species in plant–pathogen interactions. In: del Rio LA, Puppo A (eds) Reactive oxygen species in plant signaling. Springer, Berlin, pp 113–133
Bolwell GP, Robbins MP, Dixon RA (1985) Metabolic changes in elicitor-treated bean cells. Enzymic responses associated with rapid changes in cell wall components. Eur J Biochem 148:571–578
Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23:517–532
Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM (1998) Comparative biochemistry of the oxidative burst produced by rose and french bean cells reveals two distinct mechanisms. Plant Phys 116:1379–1385
Bolwell GP, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F, Rowntree EG, Wojtaszek P (1999) Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radic Res 31(Suppl):S137–S145
Bolwell PP, Page A, Pislewska M, Wojtaszek P (2001) Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma 217:20–32
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53:1367–1376
Brown I, Trethowan J, Kerry M, Mansfield J, Bolwell GP (1998) Localization of components of the oxidative cross-linking of glycoproteins and of callose synthesis in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestris and French bean mesophyll cells. Plant J 15:333–343
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Chandra S, Stennis M, Low PS (1997) Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem 272:28274–28280
Chaouch S, Queval G, Noctor G (2012) AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J 69:613–627
Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, Van Breusegem F, Saindrenan P, Noctor G (2010) Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol 153:1692–1705
Chasov A, Minibayeva F (2009) Effect of exogenous phenols on superoxide production by extracellular peroxidase from wheat seedling roots. Biochemistry (Moscow) 74:766–774
Chen X-Y, Kim ST, Cho WK, Rim Y, Kim S, Kim S-W, Kang KY, Park ZY, Kim J-Y (2009) Proteomics of weakly bound cell wall proteins in rice calli. J Plant Physiol 166:675–685
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Chivasa S, Ndimba BK, Simon WJ, Robertson D, Yu XL, Knox JP, Bolwell P, Slabas AR (2002) Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis 23:1754–1765
Chivasa S, Hamilton JM, Pringle RS, Ndimba BK, Simon WJ, Lindsey K, Slabas AR (2006) Proteomic analysis of differentially expressed proteins in fungal elicitor-treated Arabidopsis cell cultures. J Exp Bot 57:1553–1562
Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol 145:890–904
Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323:95–101
Conklin PL, Last RL (1995) Differential accumulation of antioxidant mRNAs in Arabidopsis thaliana exposed to ozone. Plant Physiol 109:203–212
Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell
Davies DR, Bindschedler LV, Strickland TS, Bolwell GP (2006) Production of reactive oxygen species in Arabidopsis thaliana cell suspension cultures in response to an elicitor from Fusarium oxysporum: implications for basal resistance. J Exp Bot 57:1817–1827
De Jong D, Yakimova ET, Kapchina VM, Woltering EJ (2002) A critical role for ethylene in hydrogen peroxide release during programmed cell death in tomato suspension cells. Planta 214:537–545
de Melo MP, Curi TCP, Miyasaka CK, Palanch AC, Curi R (1998) Effect of indole acetic acid on oxygen metabolism in cultured rat neutrophil. Gen Pharmacol 31:573–578
Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127:159–172
Desikan R, Hancock JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ (2005) A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol 137:831–834
Desikan R, Neill SJ, Hancock JT (2000) Hydrogen peroxide-induced gene expression in Arabidopsis thaliana. Free Radic Biol Med 28:773–778
Devlin WS, Gustine DL (1992) Involvement of the oxidative burst in phytoalexin accumulation and the hypersensitive reaction. Plant Physiol 100:1189–1195
Devoto A, Leckie F, Lupotto E, Cervone F, De Lorenzo G (1998) The promoter of a gene encoding a polygalacturonase-inhibiting protein of Phaseolus vulgaris L. is activated by wounding but not by elicitors or pathogen infection. Planta 205:165–174
Dubreuil-Maurizi C, Trouvelot S, Frettinger P, Pugin A, Wendehenne D, Poinssot BÆ (2010) β-Aminobutyric acid primes an NADPH oxidase-dependent reactive oxygen species production during grapevine-triggered immunity. Mol Plant Microbe Interact 23:1012–1021
Dunford B (1993) Kinetics of peroxides reactions: horseradish, barley, Corpinus cinereus, lignin and manganese. In: Welinder KG, Rasmussen S, Penel C, Greppin H (eds) Plant peroxidases: biochemistry and physiology. University of Geneva, Geneva, pp 113–124
Dwyer SC, Legendre L, Low PS, Leto TL (1996) Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta 1289:231–237
Enyedi AJ, Yalpani N, Silverman P, Raskin I (1992) Signal molecules in systemic plant resistance to pathogens and pests. Cell 70:879–886
Felix G, Regenass M, Boller T (1993) Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J 4:307–316
Ferrari S, Galletti R, Vairo D, Cervone F, De Lorenzo G (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol Plant Microbe Interact 19:931–936
Ferrer MA, Pedreño MA, Muñoz R, Barceló AR (1990) Oxidation of coniferyl alcohol by cell wall peroxidases at the expense of indole-3-acetic acid and O2: a model for the lignification of plant cell walls in the absence of H2O2. FEBS Lett 276:127–130
Forcat S, Bennett M, Grant M, Mansfield JW (2010) Rapid linkage of indole carboxylic acid to the plant cell wall identified as a component of basal defence in Arabidopsis against hrp mutant bacteria. Phytochemistry 71:870–876
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Frahry G, Schopfer P (1998) Inhibition of O2-reducing activity of horseradish peroxidase by diphenyleneiodonium. Phytochemistry 48:223–227
Fuhrs H, Gotze S, Specht A, Erban A, Gallien S, Heintz D, Van Dorsselaer A, Kopka J, Braun H-P, Horst WJ (2009) Characterization of leaf apoplastic peroxidases and metabolites in Vigna unguiculata in response to toxic manganese supply and silicon. J Exp Bot 60:1663–1678
Garrido I, Espinosa F, Alvarez-Tinaut M (2011) Apoplastic superoxide production and peroxidase activity by intact and excised axenically grown seedling roots of sunflower. Protoplasma:1–10
Gazaryan IG, Chubar TA, Mareeva EA, Lagrimini LM, Van Huystee RB, Thorneley RNF (1999) Aerobic oxidation of indole-3-acetic acid catalysed by anionic and cationic peanut peroxidase. Phytochemistry 51:175–186
Gleason C, Huang S, Thatcher LF, Foley RC, Anderson CR, Carroll AJ, Millar AH, Singh KB (2011) Mitochondrial complex II has a key role in mitochondrial-derived reactive oxygen species influence on plant stress gene regulation and defense. Proc Natl Acad Sci USA 108:10768–10773
Gomez-Gomez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5:1003–1011
Gross GG, Janse C (1977) Formation of NADH and hydrogen peroxide by cell wall associated enzymes from Forsythia xylem. Z Pflanzenphysiol 84:447–452
Gross GG, Janse C, Elstner EF (1977) Involvement of malate, monophenols, and the superoxide radical in hydrogen peroxide formation by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.). Planta 136:271–276
Hadži-Tašković Šukalović V, Vuletić M, Marković K, Vučinić Z (2011) Cell wall-associated malate dehydrogenase activity from maize roots. Plant Sci 181:465–470
Halliwell B (1978) Lignin synthesis: the generation of hydrogen peroxide and superoxide by horseradish peroxidase and its stimulation by manganese (II) and phenols. Planta 140:81–88
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Hardham AR, Takemoto D, White RG (2008) Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol 8:63
Hargreaves IP, Duncan AJ, Wu L, Agrawal A, Land JM, Heales SJR (2007) Inhibition of mitochondrial complex IV leads to secondary loss complex II-III activity: implications for the pathogenesis and treatment of mitochondrial encephalomyopathies. Mitochondrion 7:284–287
Haslam RP, Downie AL, Raveton M, Gallardo K, Job D, Pallett KE, John P, Parry MAJ, Coleman JOD (2003) The assessment of enriched apoplastic extracts using proteomic approaches. Ann Appl Biol 143:81–91
He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu LJ, Gong Z (2012) DEXH box RNA helicase–mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell. doi:10.1105/tpc.112.098707
He P, Shan L, Sheen J (2007) Elicitation and suppression of microbe-associated molecular pattern-triggered immunity in plant–microbe interactions. Cell Microbiol 9:1385–1396
Ito Y, Kaku H, Shibuya N (1997) Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J 12:347–356
Jacobo-Velazquez DA, Cisneros-Zevallos L (2009) Correlations of antioxidant activity against phenolic content revisited: a new approach in data analysis for food and medicinal plants. J Food Sci 74:R107–R113
Jamet E, Albenne C, Boudart G, Irshad M, Canut H, Pont-Lezica R (2008) Recent advances in plant cell wall proteomics. Proteomics 8:893–908
Jiang Y, Miles PW (1993) Generation of H2O2 during enzymic oxidation of catechin. Phytochemistry 33:29–34
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kaffarnik FA, Jones AM, Rathjen JP, Peck SC (2009) Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana. Mol Cell Proteomics 8:145–156
Kaida R, Satoh Y, Bulone V, Yamada Y, Kaku T, Hayashi T, Kaneko TS (2009) Activation of beta-glucan synthases by wall-bound purple acid phosphatase in tobacco cells. Plant Physiol 150:1822–1830
Kaida R, Serada S, Norioka N, Norioka S, Neumetzler L, Pauly M, Sampedro J, Zarra I, Hayashi T, Kaneko TS (2010) Potential role for purple acid phosphatase in the dephosphorylation of wall proteins in tobacco cells. Plant Physiol 153:603–610
Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10:255–266
Kimura S, Kaya H, Kawarazaki T, Hiraoka G, Senzaki E, Michikawa M, Kuchitsu K (2012) Protein phosphorylation is a prerequisite for the Ca(2+)-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca(2+) and reactive oxygen species. Biochim Biophys Acta 1823:398–405
Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M (1998) Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med 188:2091–2097
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:4127–4135
Kwon H-K, Yokoyama R, Nishitani K (2005) A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol 46:843–857
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Phys 48:251–275
Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC (2009) A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326:850–853
Lehtonen MT, Akita M, Frank W, Reski R, Valkonen JPT (2011) Involvement of a class III peroxidase and the mitochondrial protein TSPO in oxidative burst upon treatment of moss plants with a fungal elicitor. Mol Plant Microbe Interact 25:363–371
Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983
Li JLY, Sulaiman M, Beckett RP, Minibayeva FV (2010) Cell wall peroxidases in the liverwort Dumortiera hirsuta are responsible for extracellular superoxide production, and can display tyrosinase activity. Physiol Plant 138:474–484
Love AJ, Yun BW, Laval V, Loake GJ, Milner JJ (2005) Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol 139:935–948
Mader M, Schloss P (1979) Isolation of malate dehydrogenase from cell walls of Nicotiana tabacum. Plant Sci Lett 17(1979):75–80
Marino D, Andrio E, Danchin EG, Oger E, Gucciardo S, Lambert A, Puppo A, Pauly N (2011) A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol 189:580–592
Martinez C, Montillet J, Bresson E, Agnel JP, Dai GH, Daniel JF, Geiger JP, Nicole M (1998) Apoplastic peroxidase generates superoxide anions in cells of cotton cotyledons undergoing the hypersensitive reaction to Xanthomonas campestris pv. malvacearum Race 18. Mol Plant Microbe Interact 11:1038–1047
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397
Mehdy MC (1994) Active oxygen species in plant defense against pathogens. Plant Physiol 105:467–472
Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM (2010) Innate immune responses activated in arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22:973–990
Mithofer A, Daxberger A, Fromhold-Treu D, Ebel J (1997) Involvement of an NAD(P)H oxidase in the elicitor-inducible oxidative burst of soybean. Phytochemistry 45:1101–1107
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol 52:561–591
McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW (1999) Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J 17:523–534
Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140:249–262
Murphy M (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89:841–850
Nühse TS, Bottrill AR, Jones AME, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51:931–940
O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP (2012) A peroxidase-dependent apoplastic oxidative burst in cultured arabidopsis cells functions in MAMP-elicited defence. Plant Physiol. doi:10.1104/pp.111.190140
O’Connell RJ, Panstruga R (2006) Tete a tete inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol 171:699–718
Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283:8885–8892
Olson DL, Williksen EP, Scheeline A (1995) An experimentally based model of the peroxidase-NADH biochemical oscillator: an enzyme-mediated chemical switch. J Am Chem Soc 117:2–15
Panda SK, Yamamoto Y, Kondo H, Matsumoto H (2008) Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. C R Biol 331:597–610
Passardi F, Tognolli M, De Meyer M, Penel C, Dunand C (2006) Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 223:965–974
Peng M, Kuc J (1992) Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology 82:696–699
Pichorner H, Couperus A, Korori S, Ebermann R (1992) Plant peroxidase has a thiol oxidase function. Phytochemistry 31:3371–3376
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088
Qiu X, Lei C, Huang L, Li X, Hao H, Du Z, Wang H, Ye H, Beerhues L, Liu B (2012) Endogenous hydrogen peroxide is a key factor in the yeast extract-induced activation of biphenyl biosynthesis in cell cultures of Sorbus aucuparia. Planta 235:217–223
Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakiere B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52:640–657
Ranieri A, Castagna A, Pacini J, Baldan B, Mensuali Sodi A, Soldatini GF (2003) Early production and scavenging of hydrogen peroxide in the apoplast of sunflower plants exposed to ozone. J Exp Bot 54:2529–2540
Roach T, Beckett RP, Minibayeva FV, Colville L, Whitaker C, Chen H, Bailly C, Kranner I (2010) Extracellular superoxide production, viability and redox poise in response to desiccation in recalcitrant Castanea sativa seeds. Plant Cell Environ 33:59–75
Roelfsema MRG, Hedrich R (2002) Studying guard cells in the intact plant: modulation of stomatal movement by apoplastic factors. New Phytol 153:425–431
Savitsky PA, Gazaryan IG, Tishkov VI, Lagrimini LM, Ruzgas T, Gorton L (1999) Oxidation of indole-3-acetic acid by dioxygen catalysed by plant peroxidases: specificity for the enzyme structure. Biochem J 340(Pt 3):579–583
Scheel D (1998) Resistance response physiology and signal transduction. Curr Opin Plant Biol 1:305–310
Schwessinger B, Ronald PC (2012) Plant innate immunity: Perception of conserved microbial signatures. Annu Rev Plant Biol 63:451–482
Schwessinger B, Zipfel C (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11:389–395
Sharma YK, Davis KR (1997) The effects of ozone on antioxidant responses in plants. Free Radic Biol Med 23:480–488
Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C (1997) Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell 9:261–270
Si Y, Dane F, Rashotte A, Kang K, Singh NK (2010) Cloning and expression analysis of the Ccrboh gene encoding respiratory burst oxidase in Citrullus colocynthis and grafting onto Citrullus lanatus (watermelon). J Exp Bot 61:1635–1642
Slabas AR, Ndimba B, Simon WJ, Chivasa S (2004) Proteomic analysis of the Arabidopsis cell wall reveals unexpected proteins with new cellular locations. Biochem Soc Trans 32:524–528
Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald P (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270:1804–1806
Stone BA, Clarke AE (1992) Chemistry and biology of (1–3)-β-glucans. La Trobe University Press, Bundoora
Sutherland MW (1991) The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol 39:79–93
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Takemoto D, Tanaka A, Scott B (2007) NADPH oxidases in fungi: diverse roles of reactive oxygen species in fungal cellular differentiation. Fungal Genet Biol 44:1065–1076
Tang M, Smith CJ (2001) Elicitor induced defence responses in Medicago sativa. New Phytol 149:401–418
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194
Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8:397–403
Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99:517–522
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Turrens JF, Alexandre A, Lehninger AL (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237:408–414
Urzúa U, Kersten PJ, Vicuña R (1998) Manganese peroxidase-dependent oxidation of glyoxylic and oxalic acids synthesized by ceriporiopsis subvermispora produces extracellular hydrogen peroxide. Appl Environ Microbiol 64:68–73
Vance CP, Anderson JO, Sherwood RT (1976) Regulation of lignin formation in reed canarygrass in relation to disease resistance. Plant Physiol 57:915–919
Vance CP, Anderson JO, Sherwood RT (1976b) Soluble and cell wall peroxidases in reed canarygrass in relation to disease resistance and localized lignin formation. Plant Physiol 57:920–922
Veitch NC (2004) Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry 65:249–259
Wally O, Punja Z (2010) Enhanced disease resistance in transgenic carrot (Daucus carota L.) plants over-expressing a rice cationic peroxidase. Planta 232:1229–1239
Wojtaszek P (1997) Oxidative burst: an early plant response to pathogen infection. Biochem J 322(Pt 3):681–692
Wojtaszek P, Trethowan J, Bolwell GP (1995) Specificity in the immobilisation of cell wall proteins in response to different elicitor molecules in suspension-cultured cells of French bean (Phaseolus vulgaris L.). Plant Mol Biol 28:1075–1087
Yao N, Eisfelder BJ, Marvin J, Greenberg JT (2004) The mitochondrion—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40:596–610
Zhu J, Chen S, Alvarez S, Asirvatham VS, Schachtman DP, Wu Y, Sharp RE (2006) Cell wall proteome in the maize primary root elongation zone. I. Extraction and identification of water-soluble and lightly ionically bound proteins. Plant Physiol 140:311–325
Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125:749–760
Acknowledgments
AD and GPB were supported by BBSRC grant BB/E021166/1. JAO was supported by the Chilean National Scholarship Program for Graduate Studies. This review is dedicated to the memory of Professor G. Paul Bolwell, D.Sc., who passed away on 13th April 2012.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A contribution to the Special Issue on Metabolic Plant Biology.
Rights and permissions
About this article
Cite this article
O’Brien, J.A., Daudi, A., Butt, V.S. et al. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236, 765–779 (2012). https://doi.org/10.1007/s00425-012-1696-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1696-9