Abstract
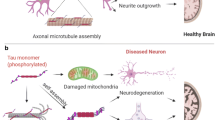
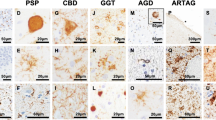
The farnesyltransferase inhibitor, Lonafarnib, reduces tau inclusions and associated atrophy in familial tauopathy models through activation of autophagy, mediated by the inhibition of farnesylation of the Ras GTPase, Rhes. While hinting at a role of Rhes in tau aggregation, it is unclear how translatable these results are for sporadic forms of tauopathy. We examined histological slides of allocortex and neocortex from multiple postmortem cases in five different tauopathies, FTLD-TDP, and healthy controls using immunofluorescence for Rhes, several tau post-translational modifications, and phospho-TDP-43. Single nucleus RNA data suggest that Rhes is found in all cortical neuron subpopulations but not in glia. Histologic investigation showed that nearly all neurons in control brains display a pattern of diffuse cytoplasmic Rhes positivity. However, in the presence of abnormal tau, but not abnormal TDP-43, the patterns of neuronal cytoplasmic Rhes tend to present as either punctiform or entirely absent. This observation reinforces the relevance of findings that link Rhes changes and tau pathology from the in vivo and in vitro models of tauopathy. The results here support a potential clinical application of Lonafarnib to tauopathies.







Similar content being viewed by others
References
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/bf00308809
Caballero B, Wang Y, Diaz A, Tasset I, Juste YR, Stiller B, Mandelkow EM, Mandelkow E, Cuervo AM (2018) Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell. https://doi.org/10.1111/acel.12692
Coughlin DG, Dickson DW, Josephs KA, Litvan I (2021) Progressive supranuclear palsy and corticobasal degeneration. Adv Exp Med Biol 1281:151–176. https://doi.org/10.1007/978-3-030-51140-1_11
DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B et al (2017) Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aag0481
Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131:1416–1432. https://doi.org/10.1093/brain/awm305
Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD et al (2012) Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 109:16666–16671. https://doi.org/10.1073/pnas.1202529109
Gordon LB, Massaro J, D’Agostino RB Sr, Campbell SE, Brazier J, Brown WT, Kleinman ME, Kieran MW, Progeria Clinical Trials C (2014) Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation 130:27–34. https://doi.org/10.1161/CIRCULATIONAHA.113.008285
Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80:1778–1783. https://doi.org/10.1212/WNL.0b013e31828726f5
Heinsen H, Strik M, Bauer M, Luther K, Ulmar G, Gangnus D, Jungkunz G, Eisenmenger W, Gotz M (1994) Cortical and striatal neurone number in Huntington’s disease. Acta Neuropathol 88:320–333. https://doi.org/10.1007/bf00310376
Hernandez I, Luna G, Rauch JN, Reis SA, Giroux M, Karch CM, Boctor D, Sibih YE, Storm NJ, Diaz A et al (2019) A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aat3005
Hochgrafe K, Sydow A, Matenia D, Cadinu D, Konen S, Petrova O, Pickhardt M, Goll P, Morellini F, Mandelkow E et al (2015) Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun 3:25. https://doi.org/10.1186/s40478-015-0204-4
Kieran MW, Packer RJ, Onar A, Blaney SM, Phillips P, Pollack IF, Geyer JR, Gururangan S, Banerjee A, Goldman S et al (2007) Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: a Pediatric Brain Tumor Consortium Study. J Clin Oncol 25:3137–3143. https://doi.org/10.1200/JCO.2006.09.4243
Kohl NE, Mosser SD, deSolms SJ, Giuliani EA, Pompliano DL, Graham SL, Smith RL, Scolnick EM, Oliff A, Gibbs JB (1993) Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260:1934–1937. https://doi.org/10.1126/science.8316833
Leng K, Li E, Eser R, Piergies A, Sit R, Tan M, Neff N, Li SH, Rodriguez RD, Suemoto CK et al (2021) Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat Neurosci 5:454. https://doi.org/10.1038/s41593-020-00764-7
Lopez A, Lee SE, Wojta K, Ramos EM, Klein E, Chen J, Boxer AL, Gorno-Tempini ML, Geschwind DH, Schlotawa L et al (2017) A152T tau allele causes neurodegeneration that can be ameliorated in a zebrafish model by autophagy induction. Brain 140:1128–1146. https://doi.org/10.1093/brain/awx005
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. https://doi.org/10.1007/s00401-011-0845-8
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. https://doi.org/10.1212/01.wnl.0000187889.17253.b1
Mead E, Kestoras D, Gibson Y, Hamilton L, Goodson R, Jones S, Eversden S, Davies P, O’Neill M, Hutton M et al (2016) Halting of caspase activity protects Tau from MC1-conformational change and aggregation. J Alzheimers Dis 54:1521–1538. https://doi.org/10.3233/JAD-150960
Mealer RG, Murray AJ, Shahani N, Subramaniam S, Snyder SH (2014) Rhes, a striatal-selective protein implicated in Huntington disease, binds beclin-1 and activates autophagy. J Biol Chem 289:3547–3554. https://doi.org/10.1074/jbc.M113.536912
Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA et al (2015) Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med 21:1154–1162. https://doi.org/10.1038/nm.3951
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Munoz DG, Dickson DW, Bergeron C, Mackenzie IR, Delacourte A, Zhukareva V (2003) The neuropathology and biochemistry of frontotemporal dementia. Ann Neurol 54(Suppl 5):S24-28. https://doi.org/10.1002/ana.10571
Nixon RA (2013) The role of autophagy in neurodegenerative disease. Nat Med 19:983–997. https://doi.org/10.1038/nm.3232
Onyike CU, Diehl-Schmid J (2013) The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25:130–137. https://doi.org/10.3109/09540261.2013.776523
Pan J, Song E, Cheng C, Lee MH, Yeung SC (2009) Farnesyltransferase inhibitors-induced autophagy: alternative mechanisms? Autophagy 5:129–131. https://doi.org/10.4161/auto.5.1.7329
Piras A, Collin L, Gruninger F, Graff C, Ronnback A (2016) Autophagic and lysosomal defects in human tauopathies: analysis of postmortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol Commun 4:22. https://doi.org/10.1186/s40478-016-0292-9
Rodriguez RD, Suemoto CK, Molina M, Nascimento CF, Leite RE, de Lucena Ferretti-Rebustini RE, Farfel JM, Heinsen H, Nitrini R, Ueda K et al (2016) Argyrophilic grain disease: demographics, clinical, and neuropathological features from a large autopsy study. J Neuropathol Exp Neurol 75:628–635. https://doi.org/10.1093/jnen/nlw034
Subramaniam S (2020) Rhes tunnels: a radical new way of communication in the Brain’s Striatum? BioEssays. https://doi.org/10.1002/bies.201900231
Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A (2011) Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci 15:191–193. https://doi.org/10.1038/nn.2994
Tacik P, Sanchez-Contreras M, Rademakers R, Dickson DW, Wszolek ZK (2016) Genetic disorders with Tau pathology: a review of the literature and report of two patients with tauopathy and positive family histories. Neurodegener Dis 16:12–21. https://doi.org/10.1159/000440840
Takada LT (2015) The genetics of monogenic frontotemporal dementia. Dement Neuropsychol 9:219–229. https://doi.org/10.1590/1980-57642015DN93000003
Theofilas P, Ehrenberg AJ, Nguy A, Thackrey JM, Dunlop S, Mejia MB, Alho AT, Paraizo Leite RE, Rodriguez RD, Suemoto CK et al (2018) Probing the correlation of neuronal loss, neurofibrillary tangles, and cell death markers across the Alzheimer’s disease Braak stages: a quantitative study in humans. Neurobiol Aging 61:1–12. https://doi.org/10.1016/j.neurobiolaging.2017.09.007
VandeVrede L, Boxer AL, Polydoro M (2020) Targeting tau: Clinical trials and novel therapeutic approaches. Neurosci Lett. https://doi.org/10.1016/j.neulet.2020.134919
Vitucci D, Di Giorgio A, Napolitano F, Pelosi B, Blasi G, Errico F, Attrotto MT, Gelao B, Fazio L, Taurisano P et al (2016) Rasd2 modulates prefronto-striatal phenotypes in humans and ‘Schizophrenia-Like Behaviors’ in mice. Neuropsychopharmacology 41:916–927. https://doi.org/10.1038/npp.2015.228
Yust-Katz S, Liu D, Yuan Y, Liu V, Kang S, Groves M, Puduvalli V, Levin V, Conrad C, Colman H et al (2013) Phase 1/1b study of lonafarnib and temozolomide in patients with recurrent or temozolomide refractory glioblastoma. Cancer 119:2747–2753. https://doi.org/10.1002/cncr.28031
Zhang X, Hernandez I, Rei D, Mair W, Laha JK, Cornwell ME, Cuny GD, Tsai LH, Steen JA, Kosik KS (2013) Diaminothiazoles modify Tau phosphorylation and improve the tauopathy in mouse models. J Biol Chem 288:22042–22056. https://doi.org/10.1074/jbc.M112.436402
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G et al (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013
Acknowledgements
The authors thank the UCSF Memory and Aging Center patients and their families for their contributions to this work. In particular, we thank those who have donated their brains to the Neurodegenerative Disease Brain Bank. We also thank the brain bank staff, without whom this work would not be possible. Additionally, we thank the Grinberg lab staff for their technical and administrative assistance with this work. Microscopy was done at the Cancer Research Lab Molecular Imaging Center at the University of California, Berkeley. The UC Berkeley Biological Faculty Research Fund provided financial support for the appropriate equipment. We thank Feather Ives and Holly Aaron, Ph.D., for their training and assistance. This study was supported by National Institute on Aging grants K24AG053435, K08AG052648, R01AG062359, F30AG066418, and R56AG057528 as well as National Institute of Neurological Disorders and Stroke grant U54NS100717-04 with additional support from Institutional grants NIH P30 AG062422 and P01AG019724, the Rainwater Charitable Foundation, and the Larry L. Hillblom Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ehrenberg, A.J., Leng, K., Letourneau, K.N. et al. Patterns of neuronal Rhes as a novel hallmark of tauopathies. Acta Neuropathol 141, 651–666 (2021). https://doi.org/10.1007/s00401-021-02279-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02279-2