Abstract
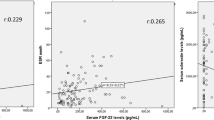
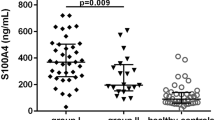
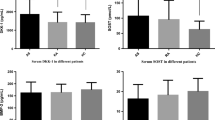
The clinical significance of C–C motif chemokine11 (CCL11) in bone metabolism in ankylosing spondylitis (AS) is not clearly elucidated. Thus, this cross-sectional study aimed to compare serum levels of CCL11 between patients with AS and healthy controls and to investigate the relationship between serum levels of CCL11 and radiographic spinal damage in patients with AS. We consecutively recruited 55 male patients with AS and 26 age- and sex-matched healthy controls. Serum levels of CCL11, tumor necrosis factor-α (TNF-α), interleukin-17, and Dickkopf-1 (DKK-1) were measured with commercially available enzyme-linked immunosorbent assay kits. Radiographs were scored according to the modified Stoke ankylosing spondylitis spine score (mSASSS), and syndesmophytes were defined as mSASSS ≥ 2. The serum levels of CCL11 in AS patients with syndesmophytes were significantly higher than those in AS patients without syndesmophytes (p = 0.007) and healthy controls (p = 0.006). In AS patients, the serum levels of CCL11 were significantly and positively correlated with mSASSS (p = 0.006), number of syndesmophytes (p = 0.029). After adjusting for confounding factors, elevated serum levels of CCL11 were associated with increased mSASSS (β = 0.007, p = 0.03) and higher risk for the presence of syndesmophytes (OR 2.34 per 50 pg/ml increase, p = 0.012) in AS patients. We found that the serum level of CCL11 was associated with structural damage in patients with AS, suggesting that CCL11 may serve as a promising biomarker for new bone formation in AS.

Similar content being viewed by others
References
Neerinckx B, Lories RJ (2017) Structural disease progression in axial spondyloarthritis: still a cause for concern? Curr Rheumatol Rep 19(3):14. https://doi.org/10.1007/s11926-017-0639-7
Park EK, Pak K, Park JH, Kim K, Kim SJ, Kim IJ, Kim GT, Lee SG (2017) Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: a 2-year longitudinal study. Rheumatol Int 37(5):765–773. https://doi.org/10.1007/s00296-017-3660-2
Neerinckx B, Lories R (2017) Mechanisms, impact and prevention of pathological bone regeneration in spondyloarthritis. Curr Opin Rheumatol 29(4):287–292. https://doi.org/10.1097/BOR.0000000000000404
Zhang M, Li XM, Wang GS, Tao JH, Chen Z, Ma Y, Li XP (2017) The association between ankylosing spondylitis and the risk of any, hip, or vertebral fracture: a meta-analysis. Medicine (Baltimore) 96(50):e8458. https://doi.org/10.1097/MD.0000000000008458
Van Mechelen M, Gulino GR, de Vlam K, Lories R (2017) Bone disease in axial spondyloarthritis. Calcif Tissue Int. https://doi.org/10.1007/s00223-017-0356-2
Sakellariou GT, Iliopoulos A, Konsta M, Kenanidis E, Potoupnis M, Tsiridis E, Gavana E, Sayegh FE (2017) Serum levels of Dkk-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Joint Bone Spine 84(3):309–315. https://doi.org/10.1016/j.jbspin.2016.05.008
Korkosz M, Gasowski J, Leszczynski P, Pawlak-Bus K, Jeka S, Kucharska E, Grodzicki T (2013) High disease activity in ankylosing spondylitis is associated with increased serum sclerostin level and decreased wingless protein-3a signaling but is not linked with greater structural damage. BMC Musculoskelet Disord 14:99. https://doi.org/10.1186/1471-2474-14-99
Kim KJ, Kim JY, Park SJ, Yoon H, Yoon CH, Kim WU, Cho CS (2012) Serum leptin levels are associated with the presence of syndesmophytes in male patients with ankylosing spondylitis. Clin Rheumatol 31(8):1231–1238. https://doi.org/10.1007/s10067-012-1999-z
Syrbe U, Callhoff J, Conrad K, Poddubnyy D, Haibel H, Junker S, Frommer KW, Muller-Ladner U, Neumann E, Sieper J (2015) Serum adipokine levels in patients with ankylosing spondylitis and their relationship to clinical parameters and radiographic spinal progression. Arthritis Rheumatol 67(3):678–685. https://doi.org/10.1002/art.38968
Park JH, Lee SG, Jeon YK, Park EK, Suh YS, Kim HO (2017) Relationship between serum adipokine levels and radiographic progression in patients with ankylosing spondylitis: a preliminary 2-year longitudinal study. Medicine (Baltimore) 96(33):e7854. https://doi.org/10.1097/MD.0000000000007854
Danve A, O’Dell J (2015) The ongoing quest for biomarkers in Ankylosing Spondylitis. Int J Rheum Dis 18(8):826–834. https://doi.org/10.1111/1756-185X.12779
Maksymowych WP (2015) Biomarkers in axial spondyloarthritis. Curr Opin Rheumatol 27(4):343–348. https://doi.org/10.1097/BOR.0000000000000180
Prajzlerova K, Grobelna K, Pavelka K, Senolt L, Filkova M (2016) An update on biomarkers in axial spondyloarthritis. Autoimmun Rev 15(6):501–509. https://doi.org/10.1016/j.autrev.2016.02.002
Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, Adcock IM, Barnes PJ, Yao X (2014) CCL11 as a potential diagnostic marker for asthma? J Asthma 51(8):847–854. https://doi.org/10.3109/02770903.2014.917659
Paplinska M, Hermanowicz-Salamon J, Nejman-Gryz P, Bialek-Gosk K, Rubinsztajn R, Arcimowicz M, Placha G, Gora J, Chazan R, Grubek-Jaworska H (2012) Expression of eotaxins in the material from nasal brushing in asthma, allergic rhinitis and COPD patients. Cytokine 60(2):393–399. https://doi.org/10.1016/j.cyto.2012.07.001
Kindstedt E, Holm CK, Sulniute R, Martinez-Carrasco I, Lundmark R, Lundberg P (2017) CCL11, a novel mediator of inflammatory bone resorption. Sci Rep 7(1):5334. https://doi.org/10.1038/s41598-017-05654-w
Rehman MQ, Beal D, Liang Y, Noronha A, Winter H, Farraye FA, Ganley-Leal L (2013) B cells secrete eotaxin-1 in human inflammatory bowel disease. Inflamm Bowel Dis 19(5):922–933. https://doi.org/10.1097/MIB.0b013e3182802950
Hueber W, Tomooka BH, Zhao X, Kidd BA, Drijfhout JW, Fries JF, van Venrooij WJ, Metzger AL, Genovese MC, Robinson WH (2007) Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis 66(6):712–719. https://doi.org/10.1136/ard.2006.054924
Syversen SW, Goll GL, Haavardsholm EA, Boyesen P, Lea T, Kvien TK (2008) A high serum level of eotaxin (CCL 11) is associated with less radiographic progression in early rheumatoid arthritis patients. Arthritis Res Ther 10(2):R28. https://doi.org/10.1186/ar2381
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, Braun J, Landewe R (2009) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 68(12):1811–1818. https://doi.org/10.1136/ard.2008.100826
Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64(1):127–129. https://doi.org/10.1136/ard.2004.020503
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42(3):467–475. https://doi.org/10.1016/j.bone.2007.11.001
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, Hanley DA, Hodsman A, Jamal SA, Kaiser SM, Kvern B, Siminoski K, Leslie WD, Osteoporosis SAC (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Can Med Assoc J 182(17):1864–1873. https://doi.org/10.1503/cmaj.100771
Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013 (vol 75, pg 392, 2013). Maturitas 76(4):387–387. https://doi.org/10.1016/j.maturitas.2013.08.006
Watts NB, Leslie WD, Foldes AJ, Miller PD (2013) 2013 international society for clinical densitometry position development conference: task force on normative databases. J Clin Densitom 16(4):472–481. https://doi.org/10.1016/j.jocd.2013.08.001
Rossini M, Viapiana O, Adami S, Idolazzi L, Fracassi E, Gatti D (2016) Focal bone involvement in inflammatory arthritis: the role of IL17. Rheumatol Int 36(4):469–482. https://doi.org/10.1007/s00296-015-3387-x
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163. https://doi.org/10.1038/nm1538
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39(4):754–766. https://doi.org/10.1016/j.bone.2006.03.017
Uluckan O, Wagner EF (2016) Role of IL-17A signalling in psoriasis and associated bone loss. Clin Exp Rheumatol 34(4 Suppl 98):17–20
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J, Yang P (2011) Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif 44(5):420–427. https://doi.org/10.1111/j.1365-2184.2011.00769.x
Osta B, Lavocat F, Eljaafari A, Miossec P (2014) Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front Immunol 5:425. https://doi.org/10.3389/fimmu.2014.00425
Croes M, Oner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, Dhert WJA, Alblas J (2016) Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 84:262–270. https://doi.org/10.1016/j.bone.2016.01.010
Arends S, Spoorenberg A, Efde M, Bos R, Leijsma MK, Bootsma H, Veeger NJ, Brouwer E, van der Veer E (2014) Higher bone turnover is related to spinal radiographic damage and low bone mineral density in ankylosing spondylitis patients with active disease: a cross-sectional analysis. PLoS ONE 9(6):e99685. https://doi.org/10.1371/journal.pone.0099685
Rankin SM, Conroy DM, Williams TJ (2000) Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today 6(1):20–27
Liu X, Zhang H, Chang X, Shen J, Zheng W, Xu Y, Wang J, Gao W, He S (2017) Upregulated expression of CCR3 in rheumatoid arthritis and CCR3-dependent activation of fibroblast-like synoviocytes. Cell Biol Toxicol 33(1):15–26. https://doi.org/10.1007/s10565-016-9356-7
Chang X, Shen J, Yang H, Xu Y, Gao W, Wang J, Zhang H, He S (2016) Upregulated expression of CCR3 in osteoarthritis and CCR3 mediated activation of fibroblast-like synoviocytes. Cytokine 77:211–219. https://doi.org/10.1016/j.cyto.2015.09.012
Kang WS, Kim YJ, Park HJ, Kim SK, Paik JW, Kim JW (2018) Association of CCL11 promoter polymorphisms with schizophrenia in a Korean population. Gene 656:80–85. https://doi.org/10.1016/j.gene.2018.02.053
Hong S, Lee EE, Martin AS, Soontornniyomkij B, Soontornniyomkij V, Achim CL, Reuter C, Irwin MR, Eyler LT, Jeste DV (2017) Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophr Res 181:63–69. https://doi.org/10.1016/j.schres.2016.09.019
Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F (2014) Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res 48(1):13–15. https://doi.org/10.1016/j.jpsychires.2013.10.007
Acknowledgements
We specially thank the late Professor Sung-Il Kim who devoted himself to education, research, and patient care in Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine (1963 to 2011).
Funding
This work was supported by the research fund of Rheumatology Research Foundation (RRF-2017-01). Also, this research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03934716).
Author information
Authors and Affiliations
Contributions
DHS: research concept and study design, performance of the tests, statistical analysis, data analysis and interpretation, and drafting the article; HJ: performance of the tests and data analysis; JSR: performance of the tests and data analysis; HNL: radiographic data analysis; EK: clinical examination, sample collection and data acquisition; JHK: data interpretation; SGL: research concept and study design, study subject recruitment, statistical analysis, data analysis and interpretation, and drafting the article, manuscript editing, substantial supervision and coordination of the study. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
The present study was approved by the Research and Ethical Review Board of Pusan National University Hospital (IRB No. 1603-005-039). All study participants provided written informed consent in accordance with the principles of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Sohn, D.H., Jeong, H., Roh, J.S. et al. Serum CCL11 level is associated with radiographic spinal damage in patients with ankylosing spondylitis. Rheumatol Int 38, 1455–1464 (2018). https://doi.org/10.1007/s00296-018-4073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4073-6