Abstract
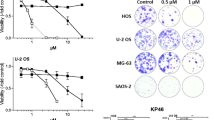
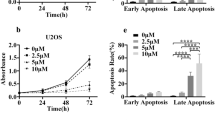
Platinum-based drugs, mainly cisplatin, are used for the treatment of several solid tumors such as OS. However, cisplatin treatment often results in the development of chemoresistance, leading therapeutic failure. We have previously reported that platinum complexes containing 8-hydroxyquinoline ligands have good antitumor activity against different cancer cell lines and with a different and better cytotoxic profile than cisplatin. Here, the anticancer properties of two different quinoline–platinum complexes [Pt(Cl)2(quinoline)(dmso)] (1) [PtCl(8-O-quinoline)(dmso)] (2) on in vitro (2D and 3D) and in vivo models (xenograft tumor of human osteosarcoma in mice) are presented. In this order, [PtCl(8-O-quinoline)(dmso)] (2) impaired cell viability to have a more pronounced antitumor effect than cisplatin on MG-63 osteosarcoma cells (IC50 4 µM vs. 39 µM). Besides, [PtCl(8-O-quinoline)(dmso)] (2) increased ROS production in a dose-manner response and this compound induced early and late apoptotic fractions of human osteosarcoma cells. Finally, [PtCl(8-O-quinoline)(dmso)] (2) decreased the cell viability of multicellular spheroids and reduced the tumor volume on athymic nude mice N:NIH(S) Fox1nu without inducing side effects. In this way, [PtCl(8-O-quinoline)(dmso)] (2) did not alter the normal cytoarchitecture of liver and kidney and the blood biomarkers (GPT, GOT, uremia, and creatinine) did not suffer modifications. Taken together, our data indicate that these compounds showed a better anticancer performance than cisplatin on in vitro and in vivo studies. These results showed the importance of chelation in the antitumor properties, suggesting that the [PtCl(8-O-quinoline)(dmso)] (2) might be a promising agent for the treatment of human osteosarcoma tumors resistant to cisplatin.






Similar content being viewed by others
References
Kelland L (2007) The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7:573–584. https://doi.org/10.1038/nrc2167
Yamamoto N, Tsuchiya H (2013) Chemotherapy for osteosarcoma—where does it come from? What is it? Where is it going? Expert Opin Pharmacother 14:2183–2193. https://doi.org/10.1517/14656566.2013.827171
Gorlick R, Khanna C (2010) Osteosarcoma. J Bone Miner Res 25:683–691. https://doi.org/10.1002/jbmr.77
Tsuchiya H, Tomita K, Mori Y et al (1999) Marginal excision for osteosarcoma with caffeine assisted chemotherapy. Clin Orthop Relat Res 358:27–35
Bode AM, Dong Z (2007) The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett 247:26–39. https://doi.org/10.1016/j.canlet.2006.03.032
Hattinger CM, Fanelli M, Tavanti E et al (2015) Advances in emerging drugs for osteosarcoma. Expert Opin Emerg Drugs. https://doi.org/10.1517/14728214.2015.1051965
Durnali A, Alkis N, Cangur S et al (2013) Prognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patients. Med Oncol 30:624. https://doi.org/10.1007/s12032-013-0624-6
Holen I, Coleman RE (2010) Bisphosphonates as treatment of bone metastases. Curr Pharm Des 16:1262–1271
McClung M, Harris ST, Miller PD et al (2013) Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med 126:13–20. https://doi.org/10.1016/j.amjmed.2012.06.023
Xue Z, Lin M, Zhu J et al (2010) Platinum(II) compounds bearing bone-targeting group: synthesis, crystal structure and antitumor activity. Chem Commun 46:1212. https://doi.org/10.1039/b922222g
Igarashi K, Yamamoto N, Hayashi K et al (2015) Effectiveness of two novel anionic and cationic platinum complexes in the treatment of osteosarcoma. Anticancer Agents Med Chem 15:390–399
Johnstone TC, Suntharalingam K, Lippard SJ (2016) The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev 116:3436–3486. https://doi.org/10.1021/acs.chemrev.5b00597
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7:1157–1178. https://doi.org/10.2147/DDDT.S49763
Afzal O, Kumar S, Haider MR et al (2015) A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem 97:871–910. https://doi.org/10.1016/j.ejmech.2014.07.044
Albrecht M, Fiege M, Osetska O (2008) 8-Hydroxyquinolines in metallosupramolecular chemistry. Coord Chem Rev 252:812–824. https://doi.org/10.1016/j.ccr.2007.06.003
Martín Santos C, Cabrera S, Ríos-Luci C et al (2013) Novel clioquinol and its analogous platinum complexes: importance, role of the halogen substitution and the hydroxyl group of the ligand. Dalton Trans 42:13343–13348. https://doi.org/10.1039/c3dt51720a
Page H, Flood P, Reynaud EG (2013) Three-dimensional tissue cultures: current trends and beyond. Cell Tissue Res 352:123–131. https://doi.org/10.1007/s00441-012-1441-5
Ho WY, Yeap SK, Ho CL et al (2012) Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PLoS One 7:e44640. https://doi.org/10.1371/journal.pone.0044640
Friedrich J, Seidel C, Ebner R, Kunz-Schughart L (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4:309–324. https://doi.org/10.1038/nprot.2008.226
Alderden RA, Mellor HR, Modok S et al (2007) Elemental tomography of cancer-cell spheroids reveals incomplete uptake of both platinum(II) and platinum(IV) complexes. J Am Chem Soc 129:13400–13401. https://doi.org/10.1021/ja076281t
Modok S, Scott R, Alderden RA et al (2007) Transport kinetics of four- and six-coordinate platinum compounds in the multicell layer tumour model. Br J Cancer 97:194–200. https://doi.org/10.1038/sj.bjc.6603854
Price JH, Williamson AN, Schramm RF, Wayland BB (1972) Palladium(II) and platinum(II) alkyl sulfoxide complexes. Examples of sulfur-bonded, mixed sulfur- and oxygen-bonded, and totally oxygen-bonded complexes. Inorg Chem 11:1280–1284. https://doi.org/10.1021/ic50112a025
Okajima T, Nakamura K, Zhang H et al (1992) Sensitive colorimetric bioassays for insulin-like growth factor (IGF) stimulation of cell proliferation and glucose consumption: use in studies of IGF analogs. Endocrinology 130:2201–2212. https://doi.org/10.1210/endo.130.4.1372238
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Butenko N, Isabel A, Nouri O et al (2009) DNA cleavage activity of V IV O (acac) 2 and derivatives. 103:622–632. https://doi.org/10.1016/j.jinorgbio.2009.01.003
Bernadou J, Pratviel G, Bennis F et al (1989) Potassium monopersulfate and a water-soluble manganese porphyrin complex, [Mn(TMPyP)](OAc)5, as an efficient reagent for the oxidative cleavage of DNA. Biochemistry 28:7268–7275
Leon IE, Di Virgilio a L, Porro V et al (2013) Antitumor properties of a vanadyl(IV) complex with the flavonoid chrysin [VO(chrysin)2EtOH]2 in a human osteosarcoma model: the role of oxidative stress and apoptosis. Dalton Trans 42:11868–11880. https://doi.org/10.1039/c3dt50524c
León IE, Cadavid-Vargas JF, Resasco A et al (2016) In vitro and in vivo antitumor effects of the VO-chrysin complex on a new three-dimensional osteosarcoma spheroids model and a xenograft tumor in mice. JBIC J Biol Inorg Chem 21:1009–1020. https://doi.org/10.1007/s00775-016-1397-0
ElBayoumi TA, Torchilin VP (2009) Tumor-targeted nanomedicines: enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res 15:1973–1980. https://doi.org/10.1158/1078-0432.CCR-08-2392
Pregosin PS (1982) Platinum-195 nuclear magnetic resonance. Coord Chem Rev 44:247–291. https://doi.org/10.1016/S0010-8545(00)80523-8
León IE, Di Virgilio AL, Barrio DA et al (2012) Hydroxylamido-amino acid complexes of oxovanadium(V). Toxicological study in cell culture and in a zebrafish model. Metallomics 4:1287–1296. https://doi.org/10.1039/c2mt20091k
Flocke LS, Trondl R, Jakupec MA, Keppler BK (2016) Molecular mode of action of NKP-1339—a clinically investigated ruthenium-based drug—involves ER- and ROS-related effects in colon carcinoma cell lines. Invest New Drugs 34:261–268. https://doi.org/10.1007/s10637-016-0337-8
Terenzi A, Pirker C, Keppler BK, Berger W (2016) Anticancer metal drugs and immunogenic cell death. J Inorg Biochem 165:71–79. https://doi.org/10.1016/j.jinorgbio.2016.06.021
Kamogashira T, Fujimoto C, Yamasoba T (2015) Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. Biomed Res Int 2015:617207. https://doi.org/10.1155/2015/617207
Slimen IB, Najar T, Ghram A et al (2014) Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia 30:513–523. https://doi.org/10.3109/02656736.2014.971446
Herr I, Debatin KM (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98:2603–2614
Mayer B, Oberbauer R (2003) Mitochondrial regulation of apoptosis. News Physiol Sci 18:89–94
Walzl A, Unger C, Kramer N et al (2014) The resazurin reduction assay can distinguish cytotoxic from cytostatic compounds in spheroid screening assays. J Biomol Screen 19:1047–1059. https://doi.org/10.1177/1087057114532352
Chen JL, Steele TWJ, Stuckey DC (2015) Modeling and application of a rapid fluorescence-based assay for biotoxicity in anaerobic digestion. Environ Sci Technol 49:13463–13471. https://doi.org/10.1021/acs.est.5b03050
Schreiber-Brynzak E, Klapproth E, Unger C et al (2015) Three-dimensional and co-culture models for preclinical evaluation of metal-based anticancer drugs. Invest New Drugs 33:835–847. https://doi.org/10.1007/s10637-015-0260-4
Shahid F, Farooqui Z, Khan F (2018) Cisplatin-induced gastrointestinal toxicity: an update on possible mechanisms and on available gastroprotective strategies. Eur J Pharmacol 827:49–57. https://doi.org/10.1016/j.ejphar.2018.03.009
Perše M, Večerić-Haler Ž (2018) Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed Res Int 2018:1–29. https://doi.org/10.1155/2018/1462802
Qin Q-P, Chen Z-F, Qin J-L et al (2015) Studies on antitumor mechanism of two planar platinum(II) complexes with 8-hydroxyquinoline: synthesis, characterization, cytotoxicity, cell cycle and apoptosis. Eur J Med Chem 92:302–313. https://doi.org/10.1016/j.ejmech.2014.12.052
Dilruba S, Kalayda GV (2016) Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol 77:1103–1124. https://doi.org/10.1007/s00280-016-2976-z
Roberts NB, Wadajkar AS, Winkles JA et al (2016) Repurposing platinum-based chemotherapies for multi-modal treatment of glioblastoma. Oncoimmunology 5:e1208876. https://doi.org/10.1080/2162402X.2016.1208876
Ruggiero A, Trombatore G, Triarico S et al (2013) Platinum compounds in children with cancer. Anticancer Drugs 24:1007–1019. https://doi.org/10.1097/CAD.0b013e3283650bda
Brozovic A, Ambriović-Ristov A, Osmak M (2010) The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol 40:347–359. https://doi.org/10.3109/10408441003601836
Fujii H, Honoki K, Tsujiuchi T et al (2009) Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol 34:1381–1386
Langdon SP (2012) Animal modeling of cancer pathology and studying tumor response to therapy. Curr Drug Targets 13:1535–1547
Acknowledgements
SBE and IEL are members of the Research Carrer, CONICET, Argentina. JFCV and MCR have a fellowship from CONICET and ANPCyT, Argentina, respectively. We also gratefully acknowledge to Dra Ortiz Mayor from Hospital Padilla, Tucuman, Argentina, to help with the Histopathology studies. Moreover, the authors would like to thank MC. Bernal for her careful revision of the manuscript.
Funding
This work was partly supported by UNLP (11X/690), CONICET (PIP 00340), and ANPCyT (PICT 2014-2223) from Argentina and CTQ2015-64561-R from Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
The animal study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, USA 2002. Laboratory Animals. A.A Tuffery, 1995. London: John Wiley). All efforts were made to minimize animal suffering, to decrease the number of animals used, and to utilize possible alternatives to in vivo techniques.
Research involving human participants
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure SM1
Effect of cisplatin on the externalization of PS by flow cytometry in MG-63 cells. Cells were incubated with 25, 50 and 100 μM of cisplatin during 6 h (A). Graphical bars show the percentage of Annexin V (+) and Annexin V (+)/ PI (+) cells. Results are expressed as the mean ± SEM, n = 9, *significant differences vs. control (p < 0.01) (B). (C) Effect of complex 2 on the MMP by flow cytometry in MG-63 cells (TIF 276 KB)
Figure SM2
Effects of cisplatin and compound 2 on survival rate. The Kaplan–Meier survival curve (A) Control vs cisplatin (B) Compound 2 vs cisplatin. The figure shows improvement of life span of xenograft-bearing mice treated compound 2 (6 mg/Kg) in comparison with cisplatin (6 mg/Kg) (n = 9 per group). Mice were treated as indicated in Figure 9 and were sacrificed throughout the study period upon reaching our study end point (TIF 74 KB)
Figure SM3
Histopathology of tumor samples. Upper panel male tumor control samples, (A) Arrow, osteoid substance, Arrow-dash, atypical osteocytes cells, Circle, necrosis zone. (B) Tumor coagulative necrosis. Lower panel male tumor treatment samples. (C) Arrow, fibrotic tissue. (D) Circles, apoptotic focuses. Tumor samples were dissected by scalpel and stained with hematoxylin/eosin. Magnifications ×40, and ×100 are indicated (TIF 1161 KB)
Figure SM4
Histopathology of the liver (A, B, D, E) and kidney samples (C, F). Upper panel male liver and kidney control samples, (A) Control liver architecture (B) Control liver Arrow: sinusoids, Dashed arrow: kupffer cells. (C) Control kidney architecture, Arrow: glomeruli, Dash arrow: tubules. Lower panel liver and kidney treatment samples (D) Treatment liver Arrow: microvacuolar (E) Treatment liver Arrow: Councilman hyaline bodies. (F) Treatment kidney, Arrow: glomeruli, Dash arrow: tubules. Samples were stained with hematoxylin/eosin. Magnifications ×40, and ×100 are indicated (TIF 1708 KB)
Rights and permissions
About this article
Cite this article
Ruiz, M.C., Resasco, A., Di Virgilio, A.L. et al. In vitro and in vivo anticancer effects of two quinoline–platinum(II) complexes on human osteosarcoma models. Cancer Chemother Pharmacol 83, 681–692 (2019). https://doi.org/10.1007/s00280-019-03773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03773-x