Abstract
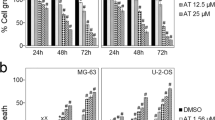
Osteosarcoma (OS) is the most common primary tumor of bone, occurring predominantly in the second decade of life. High-dose cytotoxic chemotherapy and surgical resection have improved prognosis, with long-term survival for patients with localized disease. Vanadium is an ultra-trace element that after being absorbed accumulates in bone. Besides, vanadium compounds have been studied during recent years to be considered as representative of a new class of non-platinum antitumor agents. Moreover, flavonoids are a wide family of polyphenolic compounds that display many interesting biological effects. Since coordination of ligands to metals can improve the pharmacological properties, we report herein, for the first time, the in vitro and in vivo effects of an oxidovanadium(IV) complex with the flavonoid chrysin on the new 3D human osteosarcoma and xenograft osteosarcoma mice models. The pharmacological results show that VOchrys inhibited the cell viability affecting the shape and volume of the spheroids and VOchrys suppressed MG-63 tumor growth in the nude mice without inducing toxicity and side effects. As a whole, the results presented herein demonstrate that the antitumor action of the complex was very promissory on human osteosarcoma models, whereby suggesting that VOchrys is a potentially good candidate for future use in alternative antitumor treatments.
Graphical Abstract








Similar content being viewed by others
References
Nielsen FH (1995) Metal ions in biological systems: volume 31: vanadium and its role for life. CRC Press, Boca Raton
Willsky GR, Chi LH, Godzala M et al (2011) Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord Chem Rev 255:2258–2269. doi:10.1016/j.ccr.2011.06.015
Thompson KH, Orvig C (2006) Metal complexes in medicinal chemistry: new vistas and challenges in drug design. Dalton Trans. doi:10.1039/b513476e
Thompson KH, Lichter J, LeBel C et al (2009) Vanadium treatment of type 2 diabetes: a view to the future. J Inorg Biochem 103:554–558. doi:10.1016/j.jinorgbio.2008.12.003
Etcheverry SB, Barrio DA (2007) Vanadium and bone: relevance of vanadium compounds in bone cells. In: Kustin K, Costa Pesoa J, Crans DC (eds) Vanadium: the versatile metal, chap 15, vol 974. American chemical society series, pp 204–216
Evangelou AM (2002) Vanadium in cancer treatment. Crit Rev Oncol Hematol 42:249–265
Kioseoglou E, Petanidis S, Gabriel C, Salifoglou A (2015) The chemistry and biology of vanadium compounds in cancer therapeutics. Coord Chem Rev. doi:10.1016/j.ccr.2015.03.010
Rehder D (2012) The potentiality of vanadium in medicinal applications. Future Med Chem 4:1823–1837. doi:10.4155/fmc.12.103
Pessoa JC, Etcheverry S, Gambino D (2014) Vanadium compounds in medicine. Coord Chem Rev. doi:10.1016/j.ccr.2014.12.002
Gorlick R, Khanna C (2010) Osteosarcoma. J Bone Miner Res 25:683–691. doi:10.1002/jbmr.77
Page H, Flood P, Reynaud EG (2013) Three-dimensional tissue cultures: current trends and beyond. Cell Tissue Res 352:123–131. doi:10.1007/s00441-012-1441-5
Ho WY, Yeap SK, Ho CL et al (2012) Development of multicellular tumor spheroid (MCTS) culture from breast cancer cell and a high throughput screening method using the MTT assay. PLoS One 7:e44640. doi:10.1371/journal.pone.0044640
Benien P, Swami A (2014) 3D tumor models: history, advances and future perspectives. Future Oncol 10:1311–1327. doi:10.2217/fon.13.274
Friedrich J, Eder W, Castaneda J et al (2007) A reliable tool to determine cell viability in complex 3-d culture: the acid phosphatase assay. J Biomol Screen 12:925–937. doi:10.1177/1087057107306839
Modok S, Scott R, Alderden RA et al (2007) Transport kinetics of four- and six-coordinate platinum compounds in the multicell layer tumour model. Br J Cancer 97:194–200. doi:10.1038/sj.bjc.6603854
Alderden RA, Mellor HR, Modok S et al (2007) Elemental tomography of cancer-cell spheroids reveals incomplete uptake of both platinum(II) and platinum(IV) complexes. J Am Chem Soc 129:13400–13401. doi:10.1021/ja076281t
Zhang JZ, Bryce NS, Siegele R et al (2012) The use of spectroscopic imaging and mapping techniques in the characterisation and study of DLD-1 cell spheroid tumour models. Integr Biol (Camb) 4:1072–1080. doi:10.1039/c2ib20121f
Céspedes MV, Casanova I, Parreño M, Mangues R (2006) Mouse models in oncogenesis and cancer therapy. Clin Transl Oncol 8:318–329
Francia G, Cruz-Munoz W, Man S et al (2011) Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer 11:135–141. doi:10.1038/nrc3001
Naso L, Ferrer EG, Lezama L et al (2010) Role of oxidative stress in the antitumoral action of a new vanadyl(IV) complex with the flavonoid chrysin in two osteoblast cell lines: relationship with the radical scavenger activity. J Biol Inorg Chem 15:889–902. doi:10.1007/s00775-010-0652-z
Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nat Protoc 4:309–324. doi:10.1038/nprot.2008.226
Hirschhaeuser F, Menne H, Dittfeld C et al (2010) Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 148:3–15. doi:10.1016/j.jbiotec.2010.01.012
National Research Council (2011) Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, D.C.
Crans DC, Woll KA, Prusinskas K et al (2013) Metal speciation in health and medicine represented by iron and vanadium. Inorg Chem 52:12262–12275. doi:10.1021/ic4007873
Sanna D, Ugone V, Lubinu G et al (2014) Behavior of the potential antitumor VIVO complexes formed by flavonoid ligands. 1. Coordination modes and geometry in solution and at the physiological pH. J Inorg Biochem 140:173–184. doi:10.1016/j.jinorgbio.2014.07.007
Sanna D, Micera G, Garribba E (2010) New developments in the comprehension of the biotransformation and transport of insulin-enhancing vanadium compounds in the blood serum. Inorg Chem 49:174–187. doi:10.1021/ic9017213
Thoma CR, Zimmermann M, Agarkova I et al (2014) 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev 69–70:29–41. doi:10.1016/j.addr.2014.03.001
Schreiber-Brynzak E, Klapproth E, Unger C et al (2015) Three-dimensional and co-culture models for preclinical evaluation of metal-based anticancer drugs. Invest New Drugs 33:835–847. doi:10.1007/s10637-015-0260-4
Ren L, Mendoza A, Zhu J et al (2015) Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget 6:29469–29481
Luetke A, Meyers PA, Lewis I, Juergens H (2014) Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev 40:523–532. doi:10.1016/j.ctrv.2013.11.006
Isakoff MS, Bielack SS, Meltzer P, Gorlick R (2015) Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. doi:10.1200/JCO.2014.59.4895
Leon IE, Di Virgilio AL, Porro V et al (2013) Antitumor properties of a vanadyl(IV) complex with the flavonoid chrysin [VO(chrysin)2EtOH]2 in a human osteosarcoma model: the role of oxidative stress and apoptosis. Dalton Trans 42:11868–11880. doi:10.1039/c3dt50524c
Beecher GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 133:3248S–3254S
Jafari S, Saeidnia S, Abdollahi M (2014) Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr Pharm Biotechnol 15:409–421
Reytman L, Braitbard O, Hochman J, Tshuva EY (2015) Highly effective and hydrolytically stable vanadium(V) amino phenolato antitumor agents, pp 1–9. doi:10.1021/acs.inorgchem.5b02519
León IE, Cadavid-Vargas JF, Tiscornia I et al (2015) Oxidovanadium(IV) complexes with chrysin and silibinin: anticancer activity and mechanisms of action in a human colon adenocarcinoma model. J Biol Inorg Chem 20:1175–1191. doi:10.1007/s00775-015-1298-7
Mohseny AB, Hogendoorn PCW, Cleton-Jansen A-M (2012) Osteosarcoma models: from cell lines to zebrafish. Sarcoma 2012:417271. doi:10.1155/2012/417271
Kelm JM, Timmins NE, Brown CJ et al (2003) Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 83:173–180. doi:10.1002/bit.10655
Santini M, Rainaldi G, Indovina P (2000) Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol 36:75–87
Santini MT, Rainaldi G, Ferrante A et al (2006) A 50 Hz sinusoidal magnetic field does not damage MG-63 three-dimensional tumor spheroids but induces changes in their invasive properties. Bioelectromagnetics 27:132–141. doi:10.1002/bem.20184
Bjørge L, Junnikkala S, Kristoffersen EK et al (1997) Resistance of ovarian teratocarcinoma cell spheroids to complement-mediated lysis. Br J Cancer 75:1247–1255
Vinci M, Gowan S, Boxall F et al (2012) Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol 10:29. doi:10.1186/1741-7007-10-29
Liu W-D, Zhang T, Wang C-L et al (2012) Sphere-forming tumor cells possess stem-like properties in human fibrosarcoma primary tumors and cell lines. Oncol Lett 4:1315–1320. doi:10.3892/ol.2012.940
Fujii H, Honoki K, Tsujiuchi T et al (2009) Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol 34:1381–1386
Wen Z, Liao Q, Hu Y et al (2013) A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 46:634–642. doi:10.1590/1414-431X20132647
Langdon SP (2012) Animal modeling of cancer pathology and studying tumor response to therapy. Curr Drug Targets 13:1535–1547
Ek ETH, Dass CR, Choong PFM (2006) Commonly used mouse models of osteosarcoma. Crit Rev Oncol Hematol 60:1–8. doi:10.1016/j.critrevonc.2006.03.006
El-Naggar MM, El-Waseef AM, El-Halafawy KM, El-Sayed IH (1998) Antitumor activities of vanadium(IV), manganese(IV), iron(III), cobalt(II) and copper(II) complexes of 2-methylaminopyridine. Cancer Lett 133:71–76
Köpf-Maier P (1982) Development of necroses, virus activation and giant cell formation after treatment of Ehrlich ascites tumor with metallocene dichlorides. J Cancer Res Clin Oncol 103:145–164
Samanta S, Swamy V, Suresh D et al (2008) Protective effects of vanadium against DMH-induced genotoxicity and carcinogenesis in rat colon: removal of O(6)-methylguanine DNA adducts, p53 expression, inducible nitric oxide synthase downregulation and apoptotic induction. Mutat Res 650:123–131. doi:10.1016/j.mrgentox.2007.11.001
Chakraborty T, Ghosh S, Datta S et al (2003) Vanadium suppresses sister-chromatid exchange and DNA-protein crosslink formation and restores antioxidant status and hepatocellular architecture during 2-acetylaminofluorene-induced experimental rat hepatocarcinogenesis. J Exp Ther Oncol 3:346–62
Bishayee A, Chatterjee M (1994) Inhibition of altered liver cell foci and persistent nodule growth by vanadium during diethylnitrosamine-induced hepatocarcinogenesis in rats. Anticancer Res 15:455–61
Wu Y, Ma Y, Xu Z et al (2014) Sodium orthovanadate inhibits growth of human hepatocellular carcinoma cells in vitro and in an orthotopic model in vivo. Cancer Lett 351:108–116. doi:10.1016/j.canlet.2014.05.018
Bishayee A, Waghray A, Patel MA, Chatterjee M (2010) Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Lett 294:1–12. doi:10.1016/j.canlet.2010.01.030
Acknowledgments
This work was partly supported by UNLP (11X/690), CONICET (PIP 1125), and ANPCyT (PICT 2014-2223, PPL2-2011-0008, and PME 2006-068) from Argentina. IEL and SBE are members of the Carrera del Investigador, CONICET, Argentina. JFCV is fellowship from CONICET, Argentina. The authors would like to thank to Prof. Dr. Adriana Massone (FCV, UNLP) for the management work with the tumor histopathology.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
León, I.E., Cadavid-Vargas, J.F., Resasco, A. et al. In vitro and in vivo antitumor effects of the VO-chrysin complex on a new three-dimensional osteosarcoma spheroids model and a xenograft tumor in mice. J Biol Inorg Chem 21, 1009–1020 (2016). https://doi.org/10.1007/s00775-016-1397-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1397-0