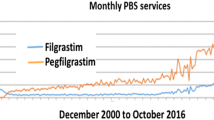
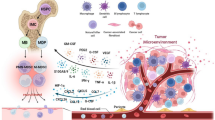
Abstract
Myeloid-derived suppressor cells (MDSCs) have been shown to contribute to tumor escape from host immune surveillance and to cancer progression by production of tumor-promoting soluble factors. Granulocyte colony-stimulating factor (G-CSF) is a principle cytokine controlling granulocyte number. Recombinant human G-CSF (rhG-CSF) has become the main therapeutic agent for the treatment of neutropenia and prophylaxis of febrile neutropenia in cancer patients. However, we show here that rhG-CSF triggers accumulation of granulocytic and monocytic subsets. Consequently, we discuss the pharmacological use of granulopoiesis stimulating factors not only in the context of febrile neutropenia but also from the perspective of MDSC-dependent and MDSC-independent mechanisms of immunosuppression and cancer angiogenesis.




Similar content being viewed by others
Abbreviations
- ASCO:
-
American Society of Clinical Oncology
- ATRA:
-
All-trans-retinoic acid
- bFGF:
-
Basic fibroblast growth factor
- C/EBPβ:
-
CCAAT/enhancer-binding protein beta
- CARS:
-
Compensatory anti-inflammatory response syndrome
- CRP:
-
C-reactive protein
- CSF:
-
Colony-stimulating factor
- DAMP:
-
Danger-associated molecular pattern
- DC:
-
Dendritic cell
- EMA:
-
European Medicines Agency
- e-MDSC:
-
Early myeloid-derived suppressor cell
- FDA:
-
Food and Drug Administration
- FN:
-
Febrile neutropenia
- HMGB1:
-
High-mobility group box 1
- IRF:
-
Interferon-regulatory factor
- M-MDSC:
-
Monocytic myeloid-derived suppressor cell
- MMP:
-
Matrix metallopeptidase
- PAMP:
-
Pathogen-associated molecular pattern
- PDGF:
-
Platelet-derived growth factor
- PEG:
-
Polyethylene glycol
- PGE2:
-
Prostaglandin E 2
- PMN-MDSC:
-
Polymorphonuclear myeloid-derived suppressor cell
- rhG-CSF:
-
Recombinant human granulocyte colony-stimulating factor
- ROS:
-
Reactive oxygen species
- TAM:
-
Tumor-associated macrophage
References
Beeson PB (1946) Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med 61:248–250
Veglia F, Perego M, Gabrilovich D (2018) Myeloid-derived suppressor cells coming of age. Nat Immunol 19:108–119. https://doi.org/10.1038/s41590-017-0022-x
Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14:302–314. https://doi.org/10.1038/nri3660
Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS (2010) STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood 116:2462–2471. https://doi.org/10.1182/blood-2009-12-259630
Talmadge JE, Gabrilovich DI (2013) History of myeloid-derived suppressor cells. Nat Rev Cancer 13:739–752. https://doi.org/10.1038/nrc3581
Shipp C, Speigl L, Janssen N, Martens A, Pawelec G (2016) A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell Mol Life Sci 73:4043–4061. https://doi.org/10.1007/s00018-016-2278-y
Najjar YG, Finke JH (2013) Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol 3:49. https://doi.org/10.3389/fonc.2013.00049
Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 22:238–244. https://doi.org/10.1016/j.coi.2010.01.021
Bronte V, Brandau S, Chen SH et al (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 7:12150. https://doi.org/10.1038/ncomms12150
Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP (1999) Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8 + T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 162:5728–5737
Waight JD, Hu Q, Miller A, Liu S, Abrams SI (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PloS One 6:e27690. https://doi.org/10.1371/journal.pone.0027690
Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101:36–47
Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE (2011) Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 186:7243–7254. https://doi.org/10.4049/jimmunol.1001952
Sakuta T, Matsushita K, Yamaguchi N et al (2001) Enhanced production of vascular endothelial growth factor by human monocytic cells stimulated with endotoxin through transcription factor SP-1. J Med Microbiol 50:233–237. https://doi.org/10.1099/0022-1317-50-3-233
Corzo CA, Condamine T, Lu L et al (2010) HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207:2439–2453. https://doi.org/10.1084/jem.20100587
Strauss L, Sangaletti S, Consonni FM et al (2015) RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell 28:253–269. https://doi.org/10.1016/j.ccell.2015.07.006
Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 69:1553–1560. https://doi.org/10.1158/0008-5472.CAN-08-1921
Solito S, Falisi E, Diaz-Montero CM et al (2011) A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 118:2254–2265. https://doi.org/10.1182/blood-2010-12-325753
Umansky V, Sevko A (2013) Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 6:169–177. https://doi.org/10.1007/s12307-012-0126-7
Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J (2013) Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 43:2943–2955. https://doi.org/10.1002/eji.201343472
Abrams SI, Waight JD (2012) Identification of a G-CSF-granulocytic MDSC axis that promotes tumor progression. Oncoimmunology 1:550–551
Allen MD, Jones LJ (2015) The role of inflammation in progression of breast cancer: friend or foe? (Review). Int J Oncol 47:797–805. https://doi.org/10.3892/ijo.2015.3075
Dorhoi A, Du Plessis N (2017) Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol 8:1895. https://doi.org/10.3389/fimmu.2017.01895
Mao Y, Poschke I, Wennerberg E et al (2013) Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73:3877–3887. https://doi.org/10.1158/0008-5472.CAN-12-4115
Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8:523–532. https://doi.org/10.1038/nri2343
Markowitz J, Wang J, Vangundy Z et al (2017) Nitric oxide mediated inhibition of antigen presentation from DCs to CD4(+) T cells in cancer and measurement of STAT1 nitration. Sci Rep 7:15424. https://doi.org/10.1038/s41598-017-14970-0
Cohen PA, Ko JS, Storkus WJ et al (2012) Myeloid-derived suppressor cells adhere to physiologic STAT3- vs STAT5-dependent hematopoietic programming, establishing diverse tumor-mediated mechanisms of immunologic escape. Immunol Investig 41:680–710. https://doi.org/10.3109/08820139.2012.703745
Shojaei F, Ferrara N (2008) Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res 68:5501–5504. https://doi.org/10.1158/0008-5472.CAN-08-0925
Ben-Baruch A (2012) The tumor-promoting flow of cells into, within and out of the tumor site: regulation by the inflammatory axis of tnfalpha and chemokines. Cancer Microenviron 5:151–164. https://doi.org/10.1007/s12307-011-0094-3
Watari K, Asano S, Shirafuji N, Kodo H, Ozawa K, Takaku F, Kamachi S (1989) Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood 73:117–122
Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, Dexter TM (1989) The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA 86:9499–9503
Sarvi F, Arabahmadi M, Alleyassin A, Aghahosseini M, Ghasemi M (2017) Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: a randomized clinical trial. Obstet Gynecol Int. 2017:3596079. https://doi.org/10.1155/2017/3596079
Huang X, Liu Y, Bai S, Peng L, Zhang B, Lu H (2017) Granulocyte colony stimulating factor therapy for stroke: a pairwise meta-analysis of randomized controlled trial. PloS One 12:e0175774. https://doi.org/10.1371/journal.pone.0175774
Kotzur T, Benavides-Garcia R, Mecklenburg J, Sanchez JR, Reilly M, Hermann BP (2017) Granulocyte colony-stimulating factor (G-CSF) promotes spermatogenic regeneration from surviving spermatogonia after high-dose alkylating chemotherapy. Reprod Biol Endocrinol 15:7. https://doi.org/10.1186/s12958-016-0226-1
Dale DC (2002) Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs 62(Suppl 1):1–15
Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, Dale DC (2011) Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117:1917–1927. https://doi.org/10.1002/cncr.25691
Yang BB, Kido A (2011) Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet 50:295–306. https://doi.org/10.2165/11586040-000000000-00000
Kuwabara T, Kobayashi S, Sugiyama Y (1996) Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev 28:625–658. https://doi.org/10.3109/03602539608994020
Carulli G (1997) Effects of recombinant human granulocyte colony-stimulating factor administration on neutrophil phenotype and functions. Haematologica 82:606–616
Tigue CC, McKoy JM, Evens AM, Trifilio SM, Tallman MS, Bennett CL (2007) Granulocyte-colony stimulating factor administration to healthy individuals and persons with chronic neutropenia or cancer: an overview of safety considerations from the Research on Adverse Drug Events and Reports project. Bone Marrow Transplant 40:185–192. https://doi.org/10.1038/sj.bmt.1705722
Socie G, Mary JY, Schrezenmeier H et al (2007) Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT). Blood 109:2794–2796. https://doi.org/10.1182/blood-2006-07-034272
Rosenberg PS, Alter BP, Bolyard AA et al (2006) The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood 107:4628–4635. https://doi.org/10.1182/blood-2005-11-4370
Hershman D, Neugut AI, Jacobson JS, Wang J, Tsai WY, McBride R, Bennett CL, Grann VR (2007) Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 99:196–205. https://doi.org/10.1093/jnci/djk028
Kim CH (2010) Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med 1:13–19. https://doi.org/10.2147/JBM.S7224
Platzbecker U, Prange-Krex G, Bornhauser M et al (2001) Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion 41:184–189
Picardi M, De Rosa G, Selleri C, Scarpato N, Soscia E, Martinelli V, Ciancia R, Rotoli B (2003) Spleen enlargement following recombinant human granulocyte colony-stimulating factor administration for peripheral blood stem cell mobilization. Haematologica 88:794–800
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N (2009) G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA 106:6742–6747. https://doi.org/10.1073/pnas.0902280106
Luyckx A, Schouppe E, Rutgeerts O et al. (2012) G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol 143:83–87. https://doi.org/10.1016/j.clim.2012.01.011
Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ (2014) G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer 110:1211–1220. https://doi.org/10.1038/bjc.2013.822
Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A (2014) A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med 3:737–746. https://doi.org/10.1002/cam4.239
Gay AN, Chang S, Rutland L, Yu L, Byeseda S, Naik-Mathuria B, Cass DL, Russell H, Olutoye OO (2008) Granulocyte colony stimulating factor alters the phenotype of neuroblastoma cells: implications for disease-free survival of high-risk patients. J Pediatr Surg 43:837–842. https://doi.org/10.1016/j.jpedsurg.2007.12.024
Rutella S, Zavala F, Danese S, Kared H, Leone G (2005) Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol 175:7085–7091
Anderlini P (2009) Effects and safety of granulocyte colony-stimulating factor in healthy volunteers. Curr Opin Hematol 16:35–40. https://doi.org/10.1097/MOH.0b013e328319913c
Pilatova K, Greplova K, Demlova R, Bencsikova B, Klement GL, Zdrazilova-Dubska L (2013) Role of platelet chemokines, PF-4 and CTAP-III, in cancer biology. J Hematol Oncol 6:42. https://doi.org/10.1186/1756-8722-6-42
Dumitru CA, Moses K, Trellakis S, Lang S, Brandau S (2012) Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol Immunother 61:1155–1167. https://doi.org/10.1007/s00262-012-1294-5
Lyman G, Reiner M, Morrow P, Crawford J (2015) The effect of filgrastim or pegfilgrastim on survival outcomes of patients with cancer receiving myelosuppressive chemotherapy. Ann Oncol 26:1452–1458. https://doi.org/10.1093/annonc/mdv174
Staar S, Rudat V, Stuetzer H, Dietz A, Volling P, Schroeder M, Flentje M, Eckel HE, Mueller RP (2001) Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 50:1161–1171
Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM (2006) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res 66:8026–8036. https://doi.org/10.1158/0008-5472.CAN-06-0158
Fishman ML, Kumar A, Davis S, Shimp W, Hrushesky WJ (2012) Guideline-based peer-to-peer consultation optimizes pegfilgrastim use with no adverse clinical consequences. J Oncol Pract 8:e14s–e17s. https://doi.org/10.1200/JOP.2012.000540
Smith TJ, Bohlke K, Lyman GH et al. (2015) Recommendations for the use of wbc growth factors: american society of clinical oncology clinical practice guideline update. J Clin Oncol 33:3199–3212. https://doi.org/10.1200/JCO.2015.62.3488
Funding
The work was supported by the Czech Ministry of Health (projects AZV 16-31966A and DRO 00209805) and the Czech Ministry of Education, Youth and Sports (projects LO1413, LM2015089, and LM2015090).
Author information
Authors and Affiliations
Contributions
KP performed or supervised laboratory testing, contributed to data interpretation, prepared figures, and drafted the manuscript. BB referred patients and drafted the manuscript. RD contributed to data interpretation and drafted the manuscript. DV drafted and edited the manuscript. LZ-D conceived of the presented idea, designed the experiment and laboratory testing, and drafted and finalized the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
For pediatric patients treated by anti-cancer DC vaccines, academic clinical trial (EudraCT: 2014-003388-39) was approved by Czech national authority (State Institute for Drug Control).
Ethical standards
For adult cancer patients, the study was approved by Ethical Board of Masaryk Memorial Cancer Institute, Brno, Czech Republic.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pilatova, K., Bencsikova, B., Demlova, R. et al. Myeloid-derived suppressor cells (MDSCs) in patients with solid tumors: considerations for granulocyte colony-stimulating factor treatment. Cancer Immunol Immunother 67, 1919–1929 (2018). https://doi.org/10.1007/s00262-018-2166-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2166-4