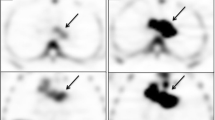
Abstract
Purpose
FMAU (1-(2′-deoxy-2′-fluoro-β-D-arabinofuranosyl)thymine) is a thymidine analog that can be phosphorylated by thymidine kinase and incorporated into DNA. This first-in-human study of [18F]FMAU was conducted as a pilot in patients to determine its biodistribution and suitability for imaging DNA synthesis in tumors using positron emission tomography (PET).
Methods
Fourteen patients with diverse cancers (brain, prostate, colorectal, lung, and breast) were imaged with [18F]FMAU. We obtained dynamic PET images for 60 min and a whole-body image. Blood and urine samples were analyzed by high-performance liquid chromatography to measure metabolites and clearance.
Results
Active tumors in the breast, brain, lung and prostate were clearly visualized with standardized uptake values (SUVs) of 2.19, 1.28, 2.21, and 2.27–4.42, respectively. Unlike with other tracers of proliferation, low uptake of [18F]FMAU was seen in the normal bone marrow (SUVmean 0.7), allowing visualization of metastatic prostate cancer (SUV 3.07). Low background was also observed in the brain, pelvis, and thorax, aside from heart uptake (SUV 3.36–8.78). In the abdomen, increased physiological uptake was seen in the liver (SUV 10.07–20.88) and kidneys (SUV 7.18–15.66) due to metabolism and/or excretion, but the urinary bladder was barely visible (SUVmean 2.03). On average, 95% of the activity in the blood was cleared within 10 min post injection and an average of 70% of the activity in the urine was intact FMAU at 60 min post injection.
Conclusion
Tumors in the brain, prostate, thorax, and bone can be clearly visualized with FMAU. In the upper abdomen, visualization is limited by the physiological uptake by the liver and kidneys.







Similar content being viewed by others
References
Shields A. Monitoring treatment response. In: Wahl R, editor. Principles and practice of positron emission tomography. Philadelphia: Lippincott; 2002. p. 252–67.
Shields AF, Mankoff DA, Link JM, Graham MM, Eary JF, Kozawa M, et al. [11C]Thymidine and FDG to measure therapy response. J Nucl Med 1998;39:1757–62.
Eary JF, Mankoff DA, Spence AM, Berger MS, Olshen A, Link JM, et al. 2-[C-11]thymidine imaging of malignant brain tumors. Cancer Res 1999;59(3):615–21.
Shields A, Grierson J, Dohmen B, Machulla H-J, Stayanoff J, Lawhorn-Crews J, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4:1334–6.
Aboagye EO, Saleem A, Cunningham VJ, Osman S, Price PM. Extraction of 5-fluorouracil by tumor and liver: a noninvasive positron emission tomography study of patients with gastrointestinal cancer. Cancer Res 2001;61(13):4937–41.
Conti P, Alauddin M, Fissekis J, Schmall B, Watanabe K. Synthesis of 2′-fluoro-5-[11C]-methyl-1-beta-D-arabinofuranosyluracil ([11C]-FMAU): a potential nucleoside analog for in vivo study of cellular proliferation with PET. Nucl Med Biol 1995;22(6):783–9.
Mangner T, Klecker R, Anderson L, Shields A. Synthesis of 2′-[18F]fluoro-2′-deoxy-β-D-arabinofuranosyl nucleotides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential pet agents for imaging cellular proliferation. Nucl Med Biol 2003(30):215–24.
Sun H, Mangner T, Collins J, Muzik O, Douglas K, Shields A. Imaging DNA synthesis in vivo with [F-18]FMAU and positron emission tomography. J Nucl Med (in press).
Fanucchi MP, Leyland-Jones B, Young CW, Burchenal JH, Watanabe KA, Fox JJ. Phase I trial of 1-(2′-deoxy-2′-fluoro-1-beta-D-arabinofuranosyl)-5-methyluracil (FMAU). Cancer Treat Rep 1985;69(1):55–9.
Abbruzzese JL, Schmidt S, Raber MN, Levy JK, Castellanos AM, Legha SS, et al. Phase I trial of 1-(2′-deoxy-2′-fluoro-1-beta-D-arabinofuranosyl)-5-methyluracil (FMAU) terminated by severe neurologic toxicity. Invest New Drugs 1989;7(2–3):195–201.
Wang H, Oliver P, Nan L, Wang S, Wang Z, Rhie JK, et al. Radiolabeled 2′-fluorodeoxyuracil-beta-D-arabinofuranoside (FAU) and 2′-fluoro-5-methyldeoxyuracil-beta-D-arabinofuranoside (FMAU) as tumor-imaging agents in mice. Cancer Chemother Pharmacol 2002;49(5):419–24.
Lewis W, Levine ES, Griniuviene B, Tankersley KO, Colacino JM, Sommadossi JP, et al. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc Natl Acad Sci U S A 1996;93(8):3592–7.
Lu L, Samuelsson L, Bergstrom M, Sato K, Fasth KJ, Langstrom B. Rat studies comparing 11C-FMAU, 18F-FLT, and 76Br-BFU as proliferation markers. J Nucl Med 2002;43(12):1688–98.
Kierdaszuk B, Krawiec K, Kazimierczuk Z, Jacobsson U, Johansson NG, Munch-Petersen B, et al. Substrate/inhibitor properties of human deoxycytidine kinase (dCK) and thymidine kinases (TK1 and TK2) towards the sugar moiety of nucleosides, including O′-alkyl analogues. Nucleosides Nucleotides 1999;18(8):1883–903.
Knecht W, Petersen GE, Munch-Petersen B, Piskur J. Deoxyribonucleoside kinases belonging to the thymidine kinase 2 (TK2)-like group vary significantly in substrate specificity, kinetics and feed-back regulation. J Mol Biol 2002;315(4):529–40.
Collins JM, Klecker RW, Katki AG. Suicide prodrugs activated by thymidylate synthase: rationale for treatment and noninvasive imaging of tumors with deoxyuridine analogues. Clin Cancer Res 1999;5(8):1976–81.
Sun H, Collins JM, Mangner TJ, Muzik O, Shields AF. Imaging [18F]FAU [1-(2′-deoxy-2′-fluoro-beta-D-arabinofuranosyl) uracil] in dogs. Nucl Med Biol 2003;30(1):25–30.
de Vries EF, van Waarde A, Harmsen MC, Mulder NH, Vaalburg W, Hospers GA. [11C]FMAU and [18F]FHPG as PET tracers for herpes simplex virus thymidine kinase enzyme activity and human cytomegalovirus infections. Nucl Med Biol 2000;27(2):113–9.
Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med 1998;39(6):990–5.
Price DT, Coleman RE, Liao RP, Robertson CN, Polascik TJ, DeGrado TR. Comparison of [18F]fluorocholine and [18F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. J Urol 2002;168(1):273–80.
Goethals P, Lameire N, van Eijkeren M, Kesteloot D, Thierens H, Dams R. [Methyl-carbon-11] thymidine for in vivo measurement of cell proliferation. J Nucl Med 1996;37(6):1048–52.
Acknowledgments
The authors thank Dr. Elizabeth Dawe for veterinary assistance, and Theresa A. Jones, CNMT, for her expert technical assistance in performing the PET studies. This work was partially supported by funding from the National Cancer Institute grants CA 83131 and CA 82645 and the U.S. Department of Defense award W81XWH-04-1-0140.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, H., Sloan, A., Mangner, T.J. et al. Imaging DNA synthesis with [18F]FMAU and positron emission tomography in patients with cancer. Eur J Nucl Med Mol Imaging 32, 15–22 (2005). https://doi.org/10.1007/s00259-004-1713-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1713-8