Summary
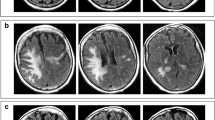
FMAU (1-[2′-deoxy-2′-fluoro-l-β-D-arabinofuranosyl]-5-methyluracil) a newly synthesized fluorinated nucleoside, has potent in vitro antiviral and antileukemic activity and is active in murine leukemia lines resistant to cytosine arabinoside. In the initial phase I trial neurotoxicity, characterized by extrapyramidal dysfunction, was found to be the dose-limiting toxic effect, and a dosage of 32 mg/m2/day for 5 days was suggested for phase II studies. We report a second phase I study of FMAU using this schedule. Mild, transient neurologic dysfunction was encountered in patients treated at the starting dose of 4 mg/m2/day × 5 days and became severe and irreversible in two patients who received the highest cumulative doses administered at the 8 mg/m2/day × 5 days level. Both severely affected patients died. Severe neurotoxicity developed and progressed in these patients despite serial neurologic examinations, including detailed neuropsychologic tests implemented in an effort to detect early neurotoxicity. Because of these findings, further study of this drug as an antileukemic agent cannot be recommended. If it is to be used as an antiviral agent, further phase I study at lower doses is advised.
Similar content being viewed by others
References
Watanabe KA, Reichman U, Hirota K, Lopez C, Fox JJ: Nucleosides 110. Synthesis and antiherpes virus activity of some 2′-fluoro-2′-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem 22:21–24, 1979
Burchenal JH, Chou T-C, Lokys L, Smith RS, Watanabe KA, Su T-L, Fox JJ: Activity of 2′-fluoro-5-methyl arabinofuranosyluracil against mouse leukemias sensitive to and resistant to 1-β-D-arabinofuranosyl cytosine. Cancer Res 42:2598–2600, 1982
Fanucchi MP, Leyland-Jones B, Young CW, Burchenal JH, Watanabe KA, Fox JJ: Phase I trial of 1-(2′-deoxy-2′-fluoro-1-β-D-arabinofuranosyl)-5-methyluracil (FMAU). Can-cer Treat Rep 69:55–59, 1985
Pirozzolo FJ, Christensen KJ, Ogle KL, Hansch EC, Thompson WG: Simple and choice reaction times in dementia: Clinical implications. Neurobiol Aging 2:113–117, 1981
Davis DD, Frenderez F, Adams F, Holmes V, Levy J, Lewis D, Neidhart J: Diagnosis of dementia in cancer patients. Physchosomatics 28:175–179, 1987
Lezak MD: Neuropsychological Assessment. Oxford University Press, New York, 1983
Castellanos AM, Fields WS: Grading neurotoxicity in cancer therapy. J Clin Oncol 4:1277–1287, 1986
Chou T-C, Burchenal JH, Schmid FA, Braun TJ, Su T-L, Watanabe KA, Fox JJ, Philips FS: Biochemical effects of 2′-fluoro-5-methyl-1-β-D-arabinofuranosyluracil and 2′-fluoro-5-iodo-1-β-D-arabinofuranosylcytosine in mouse leukemia cells sensitive and resistant to 1-β-D-arabinofuranosylcytosine. Cancer Res 42:3957–3963, 1982
Herzig RH, Hines JD, Herzig GP, Wolff SN, Cassileth PA, Lazarus HM, Adelstein DJ, Brown RA, Coccia PF, Strandjord S, Mazza JJ, Fay J, Phillips GL: Cerebellar toxicity with high-dose cytosine arabinoside. J Clin Oncol 5:927–932, 1987
Breithaupt H, Pralle H, Eckhardt T, Hattingberg MV, Schick J, Loffler H: Clinical results and pharmacokinetics of high-dose cytosine arabinoside (HD ARA-C). Cancer 50:1248–1257, 1982
Early AP, Preisler HD, Slocum H, Rustum YM: A pilot study of high-dose 1-β-D-arabinofuranosylcytosine for acute leukemia and refractory lymphoma: Clinical response and pharamacology. Cancer Res 42:1587–1594, 1982
Salinksy MC, Levine RL, Aubuchon JP, Schutta HS: Acute cerebellar dysfunction with high-dose ARA-C therapy. Cancer 51:426–429, 1983
Wolff SN, Marion J, Stein RS, Flexner JM, Lazarus HM, Spitzer TR, Phillips GL, Herzig RH, Herzig GP: High-dose cytosine arabinoside and daunorubicin as consolidation therapy for acute nonlymphocytic leukemia in first remission: A pilot study. Blood 65:1407–1411, 1985
Van Etta L, Brown J, Mastri A, Wilson T: Fatal vidarabine toxicity in a patient with normal renal function. J Am Med Assoc 246:1703–1705, 1981
Marker SC, Howard RJ, Groth KE, Mastri AR, Simmons RL, Balfour HH: A trial of vidarabine for cytomegalovirus infection in renal transplant patients. Arch Intern Med 140:1441–1444, 1980
Warrell Jr RP, Berman E: Phase I and II study of fludarabine phosphate in leukemia: Therapeutic efficacy with delayed central nervous system toxicity. J Clin Oncol 4:74–79, 1986
Merkel DE, Griffin NL, Kagen-Hallet K, Von Hoff DD: Central nervous system toxicity with fludarabine. Cancer Treat Rep 70:1449–1450, 1986
Spector R: Pharmacokinetics and metabolism of cytosine arabinoside in the central nervous system. J Pharmacol Exp Ther 222:1–6, 1982
Philips SP, Feinberg A, Chou T-G, Vidal PM, Su T-L, Watanabe KA, Fox JJ: Distribution, metabolism, and excretion of 1-(2-Fluoro-2-deoxy-β-D-arabinofuranosyl) thymine and 1-(2-Fluoro-2-deoxy-β-D-arabinofuranosyl)-5-iodocytosine. Cancer Res 43:3619–3627, 1983
Koenig H, Patel A: The acute cerebellar syndrome in 5-fluorouracil chemotherapy: a manifestation of fluoroacetate intoxication. Neurology 20:416, 1970
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abbruzzese, J.L., Schmidt, S., Raber, M.N. et al. Phase I trial of 1-(2′-deoxy-2′-fluoro-1-beta-D-arabinofuranosyl)-5-methyluracil (FMAU) terminated by severe neurologic toxicity. Invest New Drugs 7, 195–201 (1989). https://doi.org/10.1007/BF00170857
Issue Date:
DOI: https://doi.org/10.1007/BF00170857