Abstract
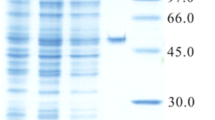
l-Leucine 5-hydroxylase (LdoA) previously found in Nostoc punctiforme PCC 73102 is a novel type of Fe(II)/α-ketoglutarate-dependent dioxygenase. LdoA catalyzed regio- and stereoselective hydroxylation of l-leucine and l-norleucine into (2S,4S)-5-hydroxyleucine and (2S)-5-hydroxynorleucine, respectively. Moreover, LdoA catalyzed sulfoxidation of l-methionine and l-ethionine in the same manner as previously described l-isoleucine 4-hydroxylase. Therefore LdoA should be a promising biocatalyst for effective production of industrially useful amino acids.
Similar content being viewed by others
References
Becker JE, Moore RE, Moore BS (2004) Cloning, sequencing, and biochemical characterization of the nostocyclopeptide biosynthetic gene cluster: molecular basis for imine macrocyclization. Gene 325:35–42
Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, Masiello P, Gomis R, Ribes G (1999) 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol Endocrinol Metab 277:40–44
Ford PW, Gustafson KR, McKee TC, Shigematsu N, Maurizi LK, Pannell LK, Williams DE, De Silva ED, Lassota P, Allen TM, Van Soest R, Andersen RJ, Boyd MR (1999) Papuamides A-D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc 121:5899–5909
Fowden L (1966) Isolation of γ-hydroxynorvaline from Lathyrus odoratus seed. Nature 209:807–808
Fowden L, Pratt HM, Smith A (1973) 4-Hydroxyisoleucine from seed of Trigonella foenum-graecum. Phytochemistry 12:1707–1711
Handa T, Yamaguchi K, Sono Y, Yazawa K (2005) Effects of fenugreek seed extract in obese mice fed a high-fat diet. Biosci Biotechnol Biochem 69:1186–1188
Hausinger RP (2004) Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol 39:21–68
Hibi M, Kawashima T, Kodera T, Smirnov SV, Sokolov PM, Sugiyama M, Shimizu S, Yokozeki K, Ogawa J (2011) Characterization of Bacillus thuringiensis l-isoleucine dioxygenase for production of useful amino acids. Appl Environ Microbiol 77:6926–6930
Hoffmann D, Hevel JM, Moore RE, Moore BS (2003) Sequence analysis and biochemical characterization of the nostopeptolide A biosynthetic gene cluster from Nostoc sp. GSV224. Gene 311:171–180
Jokela J, Herfindal L, Wahlsten M, Permi P, Selheim F, Vasconcelos V, Doskeland SO, Sivonen K (2010) A novel cyanobacterial nostocyclopeptide is a potent antitoxin against microcystins. Chembiochem 11:1594–1599
Kodera T, Smirnov SV, Samsonova NN, Kozlov YI, Koyama R, Hibi M, Ogawa J, Yokozeki K, Shimizu S (2009) A novel l-isoleucine hydroxylating enzyme, l-isoleucine dioxygenase from Bacillus thuringiensis, produces (2S,3R,4S)-4-hydroxyisoleucine. Biochem Biophys Res Commun 390:506–510
Luesch H, Hoffmann D, Hevel JM, Becker JE, Golakoti T, Moore RE (2003) Biosynthesis of 4-methylproline in cyanobacteria: cloning of nosE and nosF genes and biochemical characterization of the encoded dehydrogenase and reductase activities. J Org Chem 68:83–91
Mazmouz R, Chapuis-Hugon F, Pichon V, Mejean A, Ploux O (2011) The last step of the biosynthesis of the cyanotoxins cylindrospermopsin and 7-epi-cylindrospermopsin is catalysed by CyrI, a 2-Oxoglutarate-dependent iron oxygenase. Chembiochem 12:858–862
Mori H, Shibasaki T, Yano K, Ozaki A (1997) Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylates free l-proline to cis-3-hydroxy-L-proline. J Bacteriol 179:5677–5683
Ogawa J, Yamanaka H, Mano J, Doi Y, Horinouchi N, Kodera T, Nio N, Smirnov SV, Samsonova NN, Kozlov YI, Shimizu S (2007) Synthesis of 4-hydroxyisoleucine by the aldolase-transaminase coupling reaction and basic characterization of the aldolase from Arthrobacter simplex AKU 626. Biosci Biotechnol Biochem 71:1607–1615
Ogawa J, Kodera T, Smirnov SV, Hibi M, Samsonova NN, Koyama R, Yamanaka H, Mano J, Kawashima T, Yokozeki K, Shimizu S (2011) A novel l-isoleucine metabolism in Bacillus thuringiensis generating (2S,3R,4S)-4-hydroxyisoleucine, a potential insulinotropic and anti-obesity amino acid. Appl Microbiol Biotechnol 89:1929–1938
Ota Y, Gomi S, Yanai K (2008) Novel protein having l-leucine hydroxylase activity, useful for producing 2S,4S-5-hydroxy leucine for producing useful substance such as pharmaceutical. International Patent Application WO2008013262-A1; JP2008526830-X
Pilbeam DJ, Bell EA (1979) A reappraisal of the free amino acids in seeds of Crotalaria Juncea (Leguminosae). Phytochemistry 18:320–321
Remuzon P (1996) Trans-4-hydroxy-L-proline, a useful and versatile chiral starting block. Tetrahedron 52:13803–13835
Rippka R, Deruelles J, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sheldrick GM, Jones PG, Kennard O, Williams DH, Smith GA (1978) Structure of vancomycin and its complex with acetyl-D-alanyl-D-alanine. Nature 271:223–225
Shibasaki T, Mori H, Chiba S, Ozaki A (1999) Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microbiol 65:4028–4031
Smirnov SV, Samsonova NN, Novikova AE, Matrosov NG, Rushkevich NY, Kodera T, Ogawa J, Yamanaka H, Shimizu S (2007) A novel strategy for enzymatic synthesis of 4-hydroxyisoleucine: identification of an enzyme possessing HMKP (4-hydroxy-3-methyl-2-keto-pentanoate) aldolase activity. FEMS Microbiol Lett 273:70–77
Smirnov SV, Kodera T, Samsonova NN, Kotlyarova VA, Rushkevich NY, Kivero AD, Sokolov PM, Hibi M, Ogawa J, Shimizu S (2010) Metabolic engineering of Escherichia coli to produce (2S,3R,4S)-4-hydroxyisoleucine. Appl Microbiol Biotechnol 88:719–726
Strieker M, Kopp F, Mahlert C, Essen LO, Marahiel MA (2007) Mechanistic and structural basis of stereospecific Cβ-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem Biol 2:187–196
Yin X, Zabriskie T (2004) VioC is a non-heme iron, α-ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-hydroxy-l-arginine during viomycin biosynthesis. Chembiochem 5:1274–1277
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research (no. 21780070 and 24688010 to M. Hibi, and no. 22658027 and 23248014 to J. Ogawa) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hibi, M., Kawashima, T., Sokolov, P.M. et al. l-Leucine 5-hydroxylase of Nostoc punctiforme is a novel type of Fe(II)/α-ketoglutarate-dependent dioxygenase that is useful as a biocatalyst. Appl Microbiol Biotechnol 97, 2467–2472 (2013). https://doi.org/10.1007/s00253-012-4136-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4136-7