Abstract
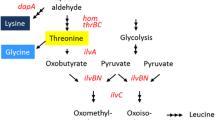
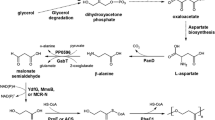
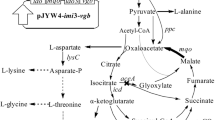
The stereo-specific l-isoleucine-4-hydroxylase (l-isoleucine dioxygenase (IDO)) was cloned and expressed in an Escherichia coli 2Δ strain lacking the activities of α-ketoglutarate dehydrogenase (EC 1.2.4.2), isocitrate liase (EC 4.1.3.1), and isocitrate dehydrogenase kinase/phosphatase (EC 2.7.11.5). The 2Δ strain could not grow in a minimal-salt/glucose/glycerol medium due to the blockage of TCA during succinate synthesis. The IDO activity in the 2Δ strain was able to “shunt” destroyed TCA, thereby coupling l-isoleucine hydroxylation and cell growth. Using this strain, we performed the direct biotransformation of l-isoleucine into 4-HIL with an 82% yield.





Similar content being viewed by others
References
Alcock NW, Crout DHG, Gregorio MVM, Lee E, Pike G, Samuel CJ (1989) Stereochemistry of the 4-hydroxyisoleucine from Trigonella foenum-graecum. Phytochemistry 28:1835–1841
Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Turnier M, Masiello P, Gomis R, Ribes G (1999) 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol Endocrinol Metab 277:617–623
Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, Rizkalla S, Pau B, Petit P, Ribes G, Ktorza A, Gross R, Reach G, Taouis M (2004) Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab 287:463–471
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
DeFronzo RA (1988) The triumvirate: β-cell, muscle, liver a collusion responsible for NIDDM. Diabetes 37:667–687
Fowden L, Pratt HM, Smith A (1973) 4-Hydroxyisoleucine from seed of Trigonella foenum graecum. Phytochemistry 12:1707–1711
Freeman H, Cox RD (2006) Type-2 diabetes: a cocktail of genetic discovery. Hum Mol Genet 15 Spec No 2:R202-9
Hausinger RP (2004) Fe(II)/α-Ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol 39:21–68
Jette L, Harvey L, Eugeny K, Levens N (2009) 4-Hydroxyisoleucine: a plant-derived treatment for metabolic syndrome. Curr Opininon Investig Drugs 10(4):353–358
Kahn SE, Porte D (1988) Islet dysfunction in non insulin-dependent diabetes mellitus. Am J Med 85:4–8
Katashkina ZhI, Skorokhodova AIu, Zimenkov DV, Gulevich AIu, Minaeva NI, Doroshenko VG, Biriukova IV, Mashko SV (2005) Tuning of expression level of the genes of interest located in the bacterial chromosome. Mol Biol (Mosk) 39(5):823–831
Kodera T, Smirnov SV, Samsonova NN, Kozlov YI, Koyama R, Hibi M, Ogawa J, Yokozeki K, Shimizu S (2009) A novel l-isoleucine hydroxylating enzyme, l-isoleucine dioxygenase from Bacillus thuringiensis, produces (2S, 3R, 4S)-4-hydroxyisoleucine. Biochem Biophys Res Commun 390(3):506–510
Narender T, Puri A, Shweta PA, Khaliq T, Saxena R, Bhatia G, Chandra R (2006) 4-Hydroxyisoleucine an unusual amino acid as antidyslipidemic and antihyperglycemic agent. Bioorg Med Chem Lett 16(2):293–296
Ogawa J, Yamanaka H, Mano J, Doi Y, Horinouchi Y, Kodera T, Nio N, Smirnov SV, Samsonova NN, Kozlov YI, Shimizu S (2007) Synthesis of 4-hydroxyisoleucine by the aldolase-transaminase coupling reaction and basic characterisation of the aldolase from Arthrobacter simplex AKU 626. Biosci Biotechnol Biochem 71:1607–1615
Ohnishi K, Hasegawa A, Matsubara K, Date T, Okada T, Kiritani K (1988) Cloning and nucleotide sequence of the brnQ gene, the structural gene for a membrane-associated component of the LIV-II transport system for branched-chain amino acids in Salmonella typhimurium. Jpn J Genet 63(4):343–357
Rolland-Fulcrand V, Rolland M, Roumestant ML, Martinez J (2004) Chemoenzymatic synthesis of enantiomerically pure (2S, 3R, 4S)-4-hydroxyisoleucine, an insulinotropic amino acid isolated from fenugreek seeds. Eur J Org Chem 873–877
Samsonova NN, Smirnov SV, Novikova AE, Ptitsyn LR (2005) Identification of Escherichia coli K12 YdcW protein as a γ-aminobutyraldehyde dehydrogenase. FEBS Lett 579:4107–4112
Sauvaire Y, Petit P, Broca C, Manteghetti M, Baissac Y, Fernandez-Alvarez J, Gross R, Roye M, Leconte A, Gomis R, Ribes G (1998) 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes 47:206–210
Smirnov SV, Samsonova NN, Novikova AE, Matrosov NG, Rushkevich NY, Kodera T, Ogawa J, Yamanaka H, Shimizu S (2007) A novel strategy for enzymatic synthesis of 4-hydroxyisoleucine: identification of an enzyme possessing HMKP (4-hydroxy-3-methyl-2-keto-pentanoate) aldolase activity. FEMS Microbiol Lett 273:70–77
Wang Q, Ouazzani J, Andre-Sasaki N, Potier P (2002) A practical synthesis of (2S, 3R, 4S)-4-hydroxyisoleucine, a potent insulinotropic a-amino acid from fenugreek. Eur J Org Chem 5:834–839
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smirnov, S.V., Kodera, T., Samsonova, N.N. et al. Metabolic engineering of Escherichia coli to produce (2S, 3R, 4S)-4-hydroxyisoleucine. Appl Microbiol Biotechnol 88, 719–726 (2010). https://doi.org/10.1007/s00253-010-2772-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2772-3