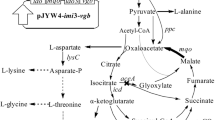
Abstract
4-Hydroxyisoleucine (HIL) found in fenugreek seeds has insulinotropic and anti-obesity effects and is expected to be a novel orally active drug for insulin-independent diabetes. Here, we show that the newly isolated strain Bacillus thuringiensis 2e2 and the closely related strain B. thuringiensis ATCC 35646 operate a novel metabolic pathway for l-isoleucine (l-Ile) via HIL and 2-amino-3-methyl-4-ketopentanoic acid (AMKP). The HIL synthesis was catalyzed stereoselectively by an α-ketoglutaric acid-dependent dioxygenase and to be useful for efficient production of a naturally occurring HIL isomer, (2S,3R,4S)-HIL. The (2S,3R,4S)-HIL was oxidized to (2S,3R)-AMKP by a NAD+-dependent dehydrogenase. The metabolic pathway functions as an effective bypass pathway that compensates for the incomplete tricarboxylic acid (TCA) cycle in Bacillus species and also explains how AMKP, a vitamin B12 antimetabolite with antibiotic activity, is synthesized. These novel findings pave a new way for the commercial production of HIL and also for AMKP.





Similar content being viewed by others
References
Alcock N, Crout D, Gregorio M, Lee E, Pike G, Samuel C (1989) Stereochemistry of the 4-hydroxyisoleucine from Trigonella foenum-graecum. Phytochemistry 28:1835–1841
Al-Habori M, Raman A (1998) Antidiabetic and hypocholesterolaemic effects of fenugreek. Phytother Res 12:233–242
Aronson J, Borris D, Doerner J, Akers E (1975) γ-Aminobutyric acid pathway and modified tricarboxylic acid cycle activity during growth and sporulation of Bacillus thuringiensis. Appl Microbiol 30:489–492
Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, Masiello P, Gomis R, Ribes G (1999) 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol Endocrinol Metab 277:40–44
Broca C, Manteghetti M, Gross R, Baissac Y, Jacob M, Petit P, Sauvaire Y, Ribes G (2000) 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur J Pharmacol 390:339–345
Chan DI, Vogel HJ (2010) Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem J 430:1–19
Fowden L, Pratt HM, Smith A (1973) 4-Hydroxyisoleucine from seed of Trigonella foenum-graecum. Phytochemistry 12:1707–1711
Haefele C, Bonfils C, Sauvaire Y (1997) Characterization of a dioxygenase from Trigonella foenum-graecum involved in 4-hydroxyisoleucine biosynthesis. Phytochemistry 44:563–566
Handa T, Yamaguchi K, Sono Y, Yazawa K (2005) Effects of fenugreek seed extract in obese mice fed a high-fat diet. Biosci Biotechnol Biochem 69:1186–1188
Hausinger RP (2004) Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol 39:21–68
Holsch K, Havel J, Haslbeck M, Weuster-Botz D (2008) Identification, cloning, and characterization of a novel ketoreductase from the cyanobacterium Synechococcus sp. strain PCC 7942. Appl Environ Microbiol 74:6697–6702
Inghardt T, Frejd T, Svensson G (1991) Organoaluminium induced ring-opening of epoxypyranosides. IV. Synthesis and structure of γ-hydroxy-lsoleucine stereoisomers and their corresponding lactones. Tetrahedron 47:6469–6482
Kodera T, Smirnov SV, Samsonova NN, Kozlov YI, Koyama R, Hibi M, Ogawa J, Yokozeki K, Shimizu S (2009) A novel l-isoleucine hydroxylating enzyme, l-isoleucine dioxygenase from Bacillus thuringiensis, produces (2S, 3R, 4S)-4-hydroxyisoleucine. Biochem Biophys Res Commun 390:506–510
Mori H, Shibasaki T, Yano K, Ozaki A (1997) Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylates free L-proline to cis-3-hydroxy-L-proline. J Bacteriol 179:5677–5683
Ogawa J, Yamanaka H, Mano J, Doi Y, Horinouchi N, Kodera T, Nio N, Smirnov SV, Samsonova NN, Kozlov YI, Shimizu S (2007) Synthesis of 4-hydroxyisoleucine by the aldolase-transaminase coupling reaction and basic characterization of the aldolase from Arthrobacter simplex AKU 626. Biosci Biotechnol Biochem 71:1607–1615
Perlman D, Perlman KL, Bodanszky M (1977) Microbial production of vitamin B12 antimetabolites. II. 2-Amino-4-keto-3-methylpentanoic acids from Bacillus cereus 439. Bioorg Chem 6:263–271
Rolland-Fulcrand V, Rolland M, Roumestant ML, Martinez J (2004) Chemoenzymatic synthesis of enantiomerically pure (2S, 3R, 4S)-4-hydroxyisoleucine, an insulinotropic amino acid isolated from fenugreek seeds. Eur J Org Chem 2004(3):873–877
Shibasaki T, Mori H, Chiba S, Ozaki A (1999) Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microbiol 65:4028–4031
Smirnov SV, Samsonova NN, Novikova AE, Matrosov NG, Rushkevich NY, Kodera T, Ogawa J, Yamanaka H, Shimizu S (2007) A novel strategy for enzymatic synthesis of 4-hydroxyisoleucine: identification of an enzyme possessing HMKP (4-hydroxy-3-methyl-2-keto-pentanoate) aldolase activity. FEMS Microbiol Lett 273:70–77
Strieker M, Kopp F, Mahlert C, Essen LO, Marahiel MA (2007) Mechanistic and structural basis of stereospecific Cβ-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem Biol 2:187–196
Wang Q, Ouazzani J, Andre-Sasaki N, Potier P (2002) A practical synthesis of (2S, 3R, 4S)-4-hydroxyisoleucine, a potent insulinotropic a-amino acid from fenugreek. Eur J Org Chem 5:834–839
Yamamoto H, Matsuyama A, Kobayashi Y (2003) Synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate using fabG-homologues. Appl Microbiol Biotechnol 61:133–139
Yin X, Zabriskie T (2004) VioC is a non-heme iron, α-ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-hydroxy-L-arginine during viomycin biosynthesis. Chembiochem 5:1274–1277
Acknowledgments
This work was partially supported by the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers (to S. Shimizu and J. Ogawa) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan, Grants-in-Aid for Scientific Research (no. 21780070 to M. Hibi), and COE for Microbial-Process Development Pioneering Future Production Systems (to S. Shimizu) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Res. Div. Microb. Sci. (J. Ogawa) is an endowed laboratory of the Institute for Fermentation, Osaka (IFO).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Ogawa, Tomohiro Kodera, Sergey V. Smirnov, and Makoto Hibi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ogawa, J., Kodera, T., Smirnov, S.V. et al. A novel l-isoleucine metabolism in Bacillus thuringiensis generating (2S,3R,4S)-4-hydroxyisoleucine, a potential insulinotropic and anti-obesity amino acid. Appl Microbiol Biotechnol 89, 1929–1938 (2011). https://doi.org/10.1007/s00253-010-2983-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2983-7