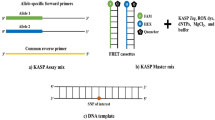
Abstract
Key message
A new time- and cost-effective strategy was developed for medium-density SNP genotyping of rice biparental populations, using GoldenGate assays based on parental resequencing.
Abstract
Since the advent of molecular markers, crop researchers and breeders have dedicated huge amounts of effort to detecting quantitative trait loci (QTL) in biparental populations for genetic analysis and marker-assisted selection (MAS). In this study, we developed a new time- and cost-effective strategy for genotyping a population of progeny from a rice cross using medium-density single nucleotide polymorphisms (SNPs). Using this strategy, 728,362 “high quality” SNPs were identified by resequencing Teqing and Lemont, the parents of the population. We selected 384 informative SNPs that were evenly distributed across the genome for genotyping the biparental population using the Illumina GoldenGate assay. 335 (87.2 %) validated SNPs were used for further genetic analyses. After removing segregation distortion markers, 321 SNPs were used for linkage map construction and QTL mapping. This strategy generated SNP markers distributed more evenly across the genome than previous SSR assays. Taking the GW5 gene that controls grain shape as an example, our strategy provided higher accuracy (0.8 Mb) and significance (LOD 5.5 and 10.1) in QTL mapping than SSR analysis. Our study thus provides a rapid and efficient strategy for genetic studies and QTL mapping using SNP genotyping assays.




Similar content being viewed by others
References
Ahn SN, Suh JP, Oh CS, Lee SJ, Suh HS (2002) Development of introgression lines of weedy rice in the background of Tongil-type rice. Rice Genet Newsl 19:14
Akhunov E, Nicolet C, Dvorak J (2009) Single nucleotide polymorphism genotyping in polyploid wheat with the Illumina GoldenGate assay. Theor Appl Genet 119:507–517
Andolfatto P, Davison D, Erezyilmaz D, Hu TT, Mast J, Sunayama-Morita T, Stern DL (2011) Multiplexed shotgun genotyping for rapid and efficient genetic mapping. Genome Res 21:610–617
Baird NA, Etter PD, Atwood TS, Currey MC, Shiver AL, Lewis ZA, Selker EU, Cresko WA, Johnson EA (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS One 3:e3376
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Cao J, Schneeberger K, Ossowski S, Gunther T, Bender S, Fitz J, Koenig D, Lanz C, Stegle O, Lippert C, Wang X, Ott F, Muller J, Alonso-Blanco C, Borgwardt K, Schmid KJ, Weigel D (2011) Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43:956–963
Chen H, He H, Zou Y, Chen W, Yu R, Liu X, Yang Y, Gao YM, Xu JL, Fan LM, Li Y, Li ZK, Deng XW (2011) Development and application of a set of breeder-friendly SNP markers for genetic analyses and molecular breeding of rice (Oryza sativa L.). Theor Appl Genet 123:869–879
Chen J, Jiang L, Wang C, Ikehashi H, Zhai HQ, Wan JM (2006) Mapping of loci for pollen sterility of indica-japonica hybrids in rice (Oryza sativa L.). Acta Agron Sin 32:515–521
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, Ramsay L, Druka A, Stein N, Svensson JT, Wanamaker S, Bozdag S, Roose ML, Moscou MJ, Chao S, Varshney RK, Szucs P, Sato K, Hayes PM, Matthews DE, Kleinhofs A, Muehlbauer GJ, DeYoung J, Marshall DF, Madishetty K, Fenton RD, Condamine P, Graner A, Waugh R (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genom 10:582
Doi K, Iwata N, Yoshimura A (1997) The construction of chromosome introgression lines of African rice (Oryza glaberrima Steud.) in the background of japonica (O. sativa L.). Rice Genet Newsl 14:39–41
Doi K, Sobrizal K, Ikeda K, Sanchez PL, Kurakazu T, Nagai Y, Yoshimura A (2003) Developing and evaluating rice chromosome segment substitution lines. Proc Int Rice Congress, pp 275–287
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6:e19379
Fan JB, Oliphant A, Shen R, Kermani BG, Garcia F, Gunderson KL, Hansen M, Steemers F, Butler SL, Deloukas P, Galver L, Hunt S, McBride C, Bibikova M, Rubano T, Chen J, Wickham E, Doucet D, Chang W, Campbell D, Zhang B, Kruglyak S, Bentley D, Haas J, Rigault P, Zhou L, Stuelpnagel J, Chee MS (2003) Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol 68:69–78
Ghesquiere A, Sequier J, Second G, Lorieux M (1997) First steps toward a rational use of African rice, Oryza glaberrima, in rice breeding: a contig line concept. Euphytica 96:31–39
Hamilton JP, Buell CR (2012) Advances in plant genome sequencing. Plant J 70:177–190
Huang X, Feng Q, Qian Q, Zhao Q, Wang L, Wang A, Guan J, Fan D, Weng Q, Huang T, Dong G, Sang T, Han B (2009) High-throughput genotyping by whole-genome resequencing. Genome Res 19:1068–1076
Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W, Guo Y, Lu Y, Zhou C, Fan D, Weng Q, Zhu C, Huang T, Zhang L, Wang Y, Feng L, Furuumi H, Kubo T, Miyabayashi T, Yuan X, Xu Q, Dong G, Zhan Q, Li C, Fujiyama A, Toyoda A, Lu T, Feng Q, Qian Q, Li J, Han B (2012a) A map of rice genome variation reveals the origin of cultivated rice. Nature 490:497–501
Huang X, Wei X, Sang T, Zhao Q, Feng Q, Zhao Y, Li C, Zhu C, Lu T, Zhang Z, Li M, Fan D, Guo Y, Wang A, Wang L, Deng L, Li W, Lu Y, Weng Q, Liu K, Huang T, Zhou T, Jing Y, Lin Z, Buckler ES, Qian Q, Zhang QF, Li J, Han B (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42:961–967
Huang X, Zhao Y, Wei X, Li C, Wang A, Zhao Q, Li W, Guo Y, Deng L, Zhu C, Fan D, Lu Y, Weng Q, Liu K, Zhou T, Jing Y, Si L, Dong G, Huang T, Lu T, Feng Q, Qian Q, Li J, Han B (2012b) Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet 44:32–39
Hyten DL, Song Q, Choi IY, Yoon MS, Specht JE, Matukumalli LK, Nelson RL, Shoemaker RC, Young ND, Cregan PB (2008) High-throughput genotyping with the GoldenGate assay in the complex genome of soybean. Theor Appl Genet 116:945–952
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079
Li H, Hearne S, Banziger M, Li Z, Wang J (2010) Statistical properties of QTL linkage mapping in biparental genetic populations. Heredity (Edinb) 105:257–267
Lu CG, Zou JS, Ikahashi H (2000) Gamete abortion locus detected by segregation distortion of isozyme locus Pgi1 in indica-japonica hybrids of rice (Oryza sativa L.). Breed Sci 50:235–239
Lu Y, Yan J, Guimaraes CT, Taba S, Hao Z, Gao S, Chen S, Li J, Zhang S, Vivek BS, Magorokosho C, Mugo S, Makumbi D, Parentoni SN, Shah T, Rong T, Crouch JH, Xu Y (2009) Molecular characterization of global maize breeding germplasm based on genome-wide single nucleotide polymorphisms. Theor Appl Genet 120:93–115
Li ZK, Fu BY, Gao YM, Xu JL, Ali J, Lafitte HR, Jiang YZ, Rey JD, Vijayakumar CH, Maghirang R, Zheng TQ, Zhu LH (2005) Genome-wide introgression lines and their use in genetic and molecular dissection of complex phenotypes in rice (Oryza sativa L.). Plant Mol Biol 59:33–52
Maughan PJ, Yourstone SM, Jellen EN, Udall JA (2009) SNP discovery via genomic reduction, barcoding and 454-pyrosequencing in Aramanth. Plant Genome 2:260–270
Maughan PJ, Yourstone SM, Byers RL, Smith SM, Udall JA (2010) Single-nucleotide polymorphism genotyping in mapping populations via genomic reduction and next-generation sequencing: proof of concept. Plant Genome 3:166–178
McCough SR, Doerge RW (1995) QTL mapping in rice. Trends Genet 11(12):482–487
McCouch SR, Kochert G, Yu ZH, Wang ZY, Khush GS, Coffman WR, Tanksley SD (1988) Molecular mapping of the rice chromosomes. Theor Appl Genet 76:815–829
Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, Childs K, Thibaud-Nissen F, Malek RL, Lee Y, Zheng L, Orvis J, Haas B, Wortman J, Buell CR (2007) The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res 35:D883–D887
Pan Q, Ali F, Yang X, Li J, Yan J (2012) Exploring the genetic characteristics of two recombinant inbred line populations via high-density SNP markers in maize. PLoS One 7:e52777
Parida SK, Mukerji M, Singh AK, Singh NK, Mohapatra T (2012) SNPs in stress-responsive rice genes: validation, genotyping, functional relevance and population structure. BMC Genomics 13:426
Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE (2012) Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS One 7:e37135
Poland JA, Brown PJ, Sorrells ME, Jannink JL (2012) Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS One 7:e32253
Saintenac C, Jiang D, Wang S, Akhunov E (2013) Sequence-based mapping of the polyploid wheat genome. G3 (Bethesda) 3:1105–1114
Smith DR, Quinlan AR, Peckham HE, Makowsky K, Tao W, Woolf B, Shen L, Donahue WF, Tusneem N, Stromberg MP, Stewart DA, Zhang L, Ranade SS, Warner JB, Lee CC, Coleman BE, Zhang Z, McLaughlin SF, Malek JA, Sorenson JM, Blanchard AP, Chapman J, Hillman D, Chen F, Rokhsar DS, McKernan KJ, Jeffries TW, Marth GT, Richardson PM (2008) Rapid whole-genome mutational profiling using next-generation sequencing technologies. Genome Res 18:1638–1642
Schouten HJ, van de Weg WE, Carling J, Khan SA, McKay SJ, van Kaauwen MP, Wittenberg AH, Koehorst-van Putten HJ, Noordijk Y, Gao Z, Rees DJ, Van Dyk MM, Jaccoud D, Considine MJ, Kilian A (2012) Diversity arrays technology (DArT) markers in apple for genetic linkage maps. Mol Breed 29:645–660
Sobrizal Ikeda K, Sanchez PL, Doi K, Angles ER, Khush GS, Yoshimura A (1999) Development of Oryza glumaepatula introgression line in rice (O. sativa L.). Rice Genet Newsl 16:107–108
Thomson MJ, Zhao K, Wright M, McNally KL, Rey J, Tung CW, Reynolds A, Scheffler B, Eizenga G, McClung A et al (2012) Highthroughput single nucleotide polymorphism genotyping for breeding applications in rice using the BeadXpress platform. Mol Breed 29:875–886
Tian F, Li DJ, Fu Q, Zhu ZF, Fu YC, Wang XK, Sun CQ (2006) Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor Appl Genet 112:570–580
van Orsouw NJ, Hogers RC, Janssen A, Yalcin F, Snoeijers S, Verstege E, Schneiders H, van der Poel H, van Oeveren J, Verstegen H, van Eijk MJ (2007) Complexity reduction of polymorphic sequences (CRoPS): a novel approach for large-scale polymorphism discovery in complex genomes. PLoS One 2:e1172
Wan J, Yanagihara S, Kato H, Ikehashi H (1993) Multiple alleles at a new locus causing hybrid sterility between a Korean indica variety and a javanica variety in rice (Oryza sativa L.). Jpn J Breed 43:507–516
Wang C, Zhu C, Zhai H, Wan J (2005) Mapping segregation distortion loci and quantitative trait loci for spikelet sterility in rice (Oryza sativa L.). Genet Res 86:97–106
Wang L, Wang A, Huang X, Zhao Q, Dong G, Qian Q, Sang T, Han B (2011) Mapping 49 quantitative trait loci at high resolution through sequencing-based genotyping of rice recombinant inbred lines. Theor Appl Genet 122:327–340
Wu KS, Tanksley SD (1993) Abundance, polymorphism and genetic mapping of microsatellites in rice. Mol Gen Genet 241:225–235
Würschum T (2012) Mapping QTL for agronomic traits in breeding populations. Theor Appl Genet 125:201–210
Xie W, Feng Q, Yu H, Huang X, Zhao Q, Xing Y, Yu S, Han B, Zhang Q (2010) Parent-independent genotyping for constructing an ultrahigh-density linkage map based on population sequencing. Proc Natl Acad Sci USA 107:10578–10583
Xu JL, Lafitte HR, Gao YM, Fu BY, Torres R, Li ZK (2005) QTLs for drought escape and tolerance identified in a set of random introgression lines of rice. Theor Appl Genet 111:1642–1650
Yamamoto T, Nagasaki H, Yonemaru J, Ebana K, Nakajima M, Shibaya T, Yano M (2010) Fine definition of the pedigree haplotypes of closely related rice cultivars by means of genome-wide discovery of single-nucleotide polymorphisms. BMC Genomics 11:267
Yu H, Xie W, Wang J, Xing Y, Xu C, Li X, Xiao J, Zhang Q (2011) Gains in QTL detection using an ultra-high density SNP map based on population sequencing relative to traditional RFLP/SSR markers. PLoS One 6:e17595
Zhao K, Wright M, Kimball J, Eizenga G, McClung A, Kovach M, Tyagi W, Ali ML, Tung CW, Reynolds A, Bustamante CD, McCouch SR (2010) Genomic diversity and introgression in O. sativa reveal the impact of domestication and breeding on the rice genome. PLoS One 5:e10780
Zheng TQ, Xu JL, Li ZK, Zhai HQ, Wan JM (2007) Genomic regions associated with milling quality and grain shape identified in a set of random introgression lines of rice (Oryza sativa L.). Plant Breeding 126:158–163
Zamir D (2001) Improving plant breeding with exotic genetic libraries. Nat Rev Genet 2:983–989
Acknowledgments
This work was supported by grants from the National Program on Key Basic Research Project of China (973 Program: 2011CB100101), Ministry of Agriculture of China (948 Program: 2011-G2B), National High Technology Research and Development Program of China (863 Program: 2012AA10A304), National Natural Science Foundation of China (U1031001) and the Program of International Science and Technology Cooperation (2012DFB32280).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
W. Chen, H. Chen and T. Zheng equally contributed to this work.
Communicated by T. Sasaki.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Supplementary material 1 (EPS 1774 kb) Workflow for the high-efficiency genotyping of a biparental population derived from a cross between Teqing and Lemont
Fig. S2
Supplementary material 2 (TIFF 3412 kb) Sequencing data of the parents of introgression lines. (a) Mapping bases (b) Genome coverage
Fig. S3
Supplementary material 3 (TIFF 478 kb). Three separate clusters of the 143 introgression lines revealed by a representative SNP. This figure was generated by the Illumina GenomeStudio Analysis Module software. The middle cluster represents heterozygous lines, while the other two clusters represent homozygous lines
Fig. S4
Supplementary material 4 (EPS 12404 kb) Genotyping of 143 introgression lines (ILs) derived from Teqing and Lemont by medium-density SNPs. (a) Red indicates genotypes consistent with Lemont, while green indicates genotypes consistent with Teqing. Yellow indicates heterozygous loci and gray means missing loci. (b) Y axis represents number of lines containing introgression fragments at each SNP locus
Fig. S5
Supplementary material 5 (TIFF 6046 kb) Comparative genotyping of IL1 (a) and IL2 (b) on the 12 chromosomes with different markers
Fig. S6
Supplementary material 6 (EPS 906 kb) Linkage map constructed by 321 SNP markers for 143 introgression lines derived from Teqing and Lemont
Fig. S7
Supplementary material 7 (TIFF 1391 kb) Comparison of QTL mapping using SNP and SSR markers of BR, MR, and HR. (a) LOD curves of QTL mapping of the brown rice ratio on chromosome 4 using 160 SSR markers. Short bars on X axis indicate the position of SSR markers. (b) LOD curves of QTL mapping of the brown rice ratio on chromosome 4 using 321 SNP markers. (c) LOD curves of QTL mapping of the milled rice rate on chromosome 4 using 160 SSR markers. (d) LOD curves of QTL mapping of the milled rice rate on chromosome 4 using all 321 SNP markers. (e) LOD curves of QTL mapping of the head rice rate on chromosome 2 using160 SSR markers. Short bars on Xaxis indicate the position of SNP markers. (f) LOD curves of QTL mapping of the head rice rate on chromosome 2 using 321 SNP markers
Fig. S8
Supplementary material 8 (TIFF 1455 kb) (a) Genotyping of progeny homozygous for GW5 delimited the locus to an ~ 0.8-Mb stretch flanked by Os05-04578273-LT and Os05-05389716-LT. Grain thickness (mean ± standard error (SE)) of 3 types of ILs. (b) Genotyping of progeny homozygous for GW5 delimited the locus to an ~ 4.4-Mb stretch flanked by RM574 and RM289
Fig. S9
Supplementary material 9 (EPS 2475 kb) Linkage map constructed by 160 genome-wide evenly selected SNP markers for 143 introgression lines derived from Teqing and Lemont
Fig. S10
Supplementary material 10 (EPS 4284 kb) QTL mapping with 160 SNP markers in comparison with 160 SSR markers. (a) LOD curves of QTL mapping of grain thickness on chromosome 5 using SSR. Short bars on X axis indicate the position of 160 SSR markers. (b) LOD curves of QTL mapping of the grain length to thickness ratio on chromosome 5 using 160 SSR markers. (c) LOD curves of QTL mapping of grain thickness on chromosome 5 using 160 SNP markers. Short bars on X axis indicate the position of 160 SNP markers. (d) LOD curves of QTL mapping of the grain length to thickness ratio on chromosome 5 using 160 SNP markers
122_2013_2218_MOESM13_ESM.xlsx
Supplementary material 13 (XLSX 170 kb) Genotyping data for Teqing-Lemont derived introgression lines.(2 means TQ,0 means Lemont,1 means heterozygous,-1 means missing)
Rights and permissions
About this article
Cite this article
Chen, W., Chen, H., Zheng, T. et al. Highly efficient genotyping of rice biparental populations by GoldenGate assays based on parental resequencing. Theor Appl Genet 127, 297–307 (2014). https://doi.org/10.1007/s00122-013-2218-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-013-2218-2