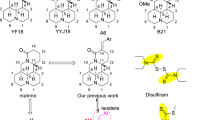
Abstract
In the present study, a series of NSAIDs–Se derivatives include selenocyanates and diselenides were synthesized and characterized, their anticancer activities against the human cancer cell lines SW480, HeLa, A549, and HepG2 were determined. Interestingly, most of the new compounds showed active in reducing the viability of different cancer lines. Compounds 1a and 1m exhibited higher promising activities than other derivatives. As the most active compound 1a showed IC50 values lower than 20 μM against the four cancer cell lines, particularly against SW480 with IC50 values below 10 μm, it shows the potential to be a promising molecular chemotherapeutic agent for colorectal cancer.

Similar content being viewed by others
References
Agrawal A, Fentiman IS (2008) NSAIDs and breast cancer: a possible prevention and treatment strategy. Int J Clin Pract 62:444–449
Aljadhey H, Tu W, Hansen RA, Blalock SJ, Brater DC, Murray MD (2012) Comparative effects of non-steroidal anti-inflammatory drugs (NSAIDs) on blood pressure in patients with hypertension. BMC Cardiovasc Disord 12:93
Arafa SA, Abdelazeem AH, Arab HH, Omar HA (2014) OSU-CG5, a novel energy restriction mimetic agent, targets human colorectal cancer cells in vitro. Acta Pharmacol Sin 35:394–400
Baines A, Taylor-Parker M, Goulet AC, Renaud C, Gerner EW, Nelson MA (2002) Selenomethionine inhibits growth and suppresses cyclooxygenase-2 (COX-2) protein expression in human colon cancer cell lines. Cancer Biol Ther 1:370–374
Bodnar M, Konieczka P, Namiesnik J (2012) The properties, functions, and use of selenium compounds in living organisms. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev 30:225–252
Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS (2007) Long-term aspirin use and mortality in women. Arch Intern Med 167:562–572
Desai D, Sinha I, Null K, Wolter W, Suckow MA, King T, Amin S, Sinha R (2010) Synthesis and antitumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int J Cancer 127:230–238
Fernandes AP, Gandin V (2015) Selenium compounds as therapeutic agents in cancer. Biochim Biophys Acta 1850:1642–1660
Ghose A, Fleming J, El-Bayoumy K, Harrison PR (2001) Enhanced sensitivity of human oral carcinomas to induction of apoptosis by selenium compounds: involvement of mitogen-activated protein kinase and Fas pathways. Cancer Res 61:7479–7487
Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT (2012) Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer 118:4768–4776
Kontogiorgis CA, Hadjipavlou-Litina DJ (2002) Non steroidal anti-inflammatory and anti-allergy agents. Curr Med Chem 9:89–98
Lee SO, Yeon Chun J, Nadiminty N, Trump DL, Ip C, Dong Y, Gao AC (2006) Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA). Prostate 66:1070–1075
Omar HA, Arafa SA, Maghrabi IA, Weng JR (2014) Sensitization of hepatocellular carcinoma cells to Apo2L/TRAIL by a novel Akt/NF-κB signalling inhibitor. Basic Clin Pharmacol Toxicol 114:464–471
Pinto JT, Sinha R, Papp K, Facompre ND, Desai D, El-Bayoumy K (2007) Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. Int J Cancer 120:1410–1417
Plano D, Baquedano Y, Moreno-Mateos D, Font M, Jimenez-Ruiz A, Palop JA, Sanmartin C (2011) Selenocyanates and diselenides: a new class of potent antileishmanial agents. Eur J Med Chem 46:3315–3323
Plano D, Karelia DN, Pandey MK, Spallholz JE, Amin S, Sharma AK (2016) Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J Med Chem 59:1946–1959
Poerschke RL, Franklin MR, Moos PJ (2008) Modulation of redox status in human lung cell lines by organoselenocompounds: selenazolidines, selenomethionine, and methylseleninic acid. Toxicol In Vitro 22:1761–1767
Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z, Meade TW (2012) Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 379:1602–1612
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomized trials. Lancet 376:1741–1750
Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK (2012) Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Intern Med 172:209–216
Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, Amin S (2008) Synthesis and anticancer activity comparison of phenylalkyl isoselenocyanates with corresponding naturally occurring and synthetic isothiocyanates. J Med Chem 51:7820–7826
Shureiqi I, Chen D, Lee JJ, Yang P, Newman RA, Brenner DE, Lotan R, Fischer SM, Lippman SM (2000) 15-LOX-1: a novel molecular target of nonsteroidal anti-inflammatory drug-induced apoptosis in colorectal cancer cells. J Natl Cancer Inst 92:1136–1142
Ulrich CM, Bigler J, Potter JD (2006) Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6:130–140
Acknowledgements
This investigation was made possible through the financial support of National Natural Science Foundation of China (Grant No: 21302065).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that caninappropriately influence our work, there is no professional or other personal interest of any nature or kind inany product, service and/or company that could be construed as influencing the position presented in, or thereview of, the manuscript entitled “Synthesis of NSAIDs–Se derivatives as potent anticancer agents”.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, l., Li, S., Li, X. et al. Synthesis of NSAIDs–Se derivatives as potent anticancer agents. Med Chem Res 27, 2071–2078 (2018). https://doi.org/10.1007/s00044-018-2216-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2216-7