Abstract
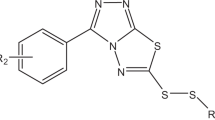
In order to obtain more effective antitumor agents, a new class of 1,2,4-triazole derivatives bearing disulfide bond were designed and synthesized. All the final compounds were confirmed by IR, 1H NMR, 13C NMR and HR-ESI-MS. The in vitro cytotoxicity of the compounds on the SMMC-7721, Hela, A549 cancer cell lines and the L929 normal cell lines were assessed by cell counting kit-8 (CCK-8). Many of tested compounds 8a–h, 9a–h, 10a–h had better cytotoxic activity on various cancer cell lines than positive control 5-fluorouracil, and they were less cytotoxic to normal cell line L929 than cancer cells. Among them, compounds 9e, 9g, and 10h showed better cytotoxic activity on SMMC-7721 cells with IC50 values 4.12, 2.92, and 4.53 μM, respectively. Compounds 8a, 9g, 10g and 10h displayed high antiproliferative activity against Hela cells with IC50 values 6.31, 4.31, 6.31 and 3.97 μM, respectively. Compounds 8c, 10a and 10h revealed effective biological potency on A549 cells with IC50 values 4.75, 4.92 and 3.73 μM, respectively. Moreover, a great majority of tested compounds revealed low cytotoxicity on normal cell line L929.


Similar content being viewed by others
References
Çoruh I, Çevik Ö, Yelekçi K, Djikic T, Küçükgüzel ŞG. Synthesis, anticancer activity, and molecular modeling of etodolac-thioether derivatives as potent methionine aminopeptidase (type II) inhibitors. Arch Pharm Chem Life Sci. 2018;351:1700195.
Mahanti S, Sunkaraa S, Bhavanib R. Synthesis, biological evaluation and computational studies of fused acridine containing 1,2,4-triazole derivatives as anticancer agents. Synth Commun. 2019;49:1729–40.
Beyzaei H, Khosravi Z, Aryan R, Ghasemi B. A green one‑pot synthesis of 3(5)‑substituted 1,2,4‑triazol‑5(3)‑amines as potential antimicrobial agents. J Iran Chem Soc. 2019;16:2565–73.
Abu-Hashem AA, Hussein HAR, Abu-zied KM. Synthesis of novel 1,2,4-triazolopyrimidines and their evaluation as antimicrobial agents. Med Chem Res. 2016;26:120–30.
Hkiri S, Hafidh A, Cavalier JF, Touil S, Samarat A. Design, synthesis, antimicrobial evaluation, and molecular docking studies of novel symmetrical 2,5-difunctionalized 1,3,4-oxadiazoles. J Heterocyl Chem. 2019;57:1044–54.
Sekhar MM, Yamini G, Divya KRG, Padmavathi V, Padmaja A. Synthesis and bioassay of a new class of disubstituted 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Med Chem Res. 2019;28:1049–62.
Bektaş H, Sökmen BB, Aydın S, Menteşe E, Bektaş A, Dilekçi G. Design, synthesis, and characterization of some new benzimidazole derivatives and biological evaluation. J Heterocyl Chem. 2019;57::2234–42.
Menteşe E, Karaali N, Yılmaz F, Ülker S, Kahveci B. Microwave-assisted synthesis and biological evaluation of some benzimidazole derivatives containing a 1,2,4-triazol ring. Arch Pharm Chem Life Sci. 2013;346:556–61.
Ozdemir SB, Demirbas N, Demirbas A, Ayaz FA, Çolak NJ. Microwave-assisted synthesis, antioxidant, and antimicrobial evaluation of piperazine-azole-fluoroquinolone based 1,2,4-triazole derivatives. J Heterocyl Chem. 2018;55:2744–59.
Doğan IS, Özdemir Z, Sari S, Bozbey I, Karakurt A, Saraç S. Synthesis, anticonvulsant activity, and molecular modeling studies of novel 1-phenyl/1-(4-chlorophenyl)-2-(1H-triazol-1-yl)ethanol ester derivatives. Med Chem Res. 2018;27:2171–86.
Kahveci B, Menteşe E, Akkaya E, Yılmaz F, Doğan İS, Özel A. Synthesis of some novel 1,2,4-triazol-3-one derivatives bearing the salicyl moiety and their anticonvulsant activities. Arch Pharm Chem Life Sci. 2014;347:449–55.
Holla BS, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur J Med Chem. 2003;38:759–67.
Al-Soud YA, Al-Masoudi NA, Ferwanah AE. Synthesis and properties of new substituted 1,2,4-triazoles: potential antitumor agents. Bioorg Med Chem. 2003;11:1701–8.
Ceylan S. Synthesis and biological evaluation of new Mannich and Schiff bases containing 1,2,4-triazole and 1,3,4-oxadiazole nucleus. Med Chem Res. 2016;25:1958–70.
Jalilian AR, Sattari S, Bineshmarvasti M, Shafiee A, Daneshtalab M. Synthesis and in vitro antifungal and cytotoxicity evaluation of thiazolo-4H-1,2,4-triazoles and 1,2,3-thiadiazolo -4H-1,2,4-triazoles. Arch Pharm. 2000;333:347–54.
Li BC, Zhang DW, Zhang YM, Jiang D, Li S, Lei W, et al. Synthesis and evaluation of novel benzene-ethanol bearing 1,2,4-triazole derivatives as potential antimicrobial agents. Med Chem Res. 2016;26:44–51.
Lingappa B, Girisha KS, Kalluraya BN, Rai S, Kumari NS. Regioselective reaction: synthesis of novel mannich bases derived from 3-(4,6-disubstituted-2-thiomethylpyrimidyl) -4-amino-5-mercapto-1,2,4-tiiazoles and their antimicrobial properties. Ind J Chem. 2008;47B:1858–64.
Zeydi MM, Montazeri N, Fouladi M. Synthesis and evaluation of novel [1,2,4]Triazolo [1,5-c] quinazoline derivatives as antibacterial agents. J Heterocyl Chem. 2017;54:3549–53.
Almajan GL, Barbuceanu SF, Almajan ER, Draghici C, Saramet G. Synthesis, characterization and antibacterial activity of some triazole Mannich bases carrying diphenylsulfone moieties. Eur J Med Chem. 2009;44:3083–9.
Abdel-Megeed AM, Abdel-Rahman HM, Alkaramany GES, El-Gendy MA. Design, synthesis and molecular modeling study of acylated 1,2,4-triazole-3-acetates with potential anti-inflammatory activity. Eur J Med Chem. 2009;44:117–23.
Sujith KV, Rao JN, Shetty P, Kalluraya B. Regioselective reaction: synthesis and pharmacological study of mannich bases containing ibuprofen moiety. Eur J Med Chem. 2009;44:3697–702.
Tozkoparan B, Aytaç SP, Aktay G. Novel 3,6-disubstituted 7H-1,2,4-triazolo[3,4-b] [1,3,4]thiadiazines: synthesis, characterization, and evaluation of analgesic/antiinflammatory, antioxidant activities. Arch Pharm Chem Life Sci. 2009;40:291–8.
Akhter MW, Hassan MZ, Amir M. Synthesis and pharmacological evaluation of 3-diphenylmethyl-6-substituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles: a condensed bridgehead nitrogen Heterocyl system. Arab J Chem. 2014;7:955–63.
Hunashal RD, Satyanarayana D, Maddi VS. Synthesis and antimicrobial activity of 2-[4-(substituted benzylidenamino)-5-(substituted phenoxymethyl)-4H-1,2,4-triazol-3-yl thio] acetic acid derivatives. E-J Chem. 2012;9:1206–12.
Ahirwar J, Ahirwar D, Lanjhiyana S, Jha AK, Dewangan D, Badwaik H. Analgesic and anti-inflammatory potential of merged pharmacophore containing 1,2,4-triazoles and substituted benzyl groups via thio linkage. J Heterocycl Chem. 2018;55:2130–41.
Miniyar PB, Mahajan AA, Mokale SN, Shah MU, Kumar AS, Chaturbhuj GU. Triazole hybrids as new type of anti-fungal agents. Arab J Chem. 2017;10:295–9.
Khanage SG, Mohite PB, Pandhare RB, Raju SA. Investigation of pyrazole and tetrazole derivatives containing 3,5 disubstituted-4H-1,2,4-triazole as a potential antitubercular and antifungal agent. Bioint Res Appl Chem. 2012;2:277–83.
Küçükgüzel I, Küçükgüzel SG, Rollas S, Kiraz M. Some 3-thioxo/alkylthio-1,2,4-triazoles with a substituted thiourea moiety as possible antimycobacterials. Bioorg Med Chem Lett. 2001;11:1703–7.
Xiao Z, Waters NC, Woodard CL, Li PK. Design and synthesis of pfmrk inhibitors as potential antimalarial agents. Bioorg Med Chem Lett. 2001;11:2875–78.
Chelamalla R, Akena V, Manda S. Synthesis of N′-arylidene-2-(5-aryl-1H-1,2,4-triazol -3-ylthio)acetohydrazides as antidepressants. Med Chem Res. 2017;26:1359–66.
Hichri F, Omri A, Hossan ASM, Jannet HB. Alpha-glucosidase and amylase inhibitory effects of eruca vesicaria subsp. longirostris essential oils: synthesis of new 1,2,4-triazole-thiol derivatives and 1,3,4-thiadiazole with potential inhibitory activity. Pharm Biol. 2019;57:564–70.
Bera H, Dolzhenko AV, Sun L, Gupta SD, Chui WK. Synthesis and in vitro evaluation of 1,2,4-triazolo[1,5-a][1,3,5]triazine derivatives as thymidine phosphorylase inhibitors. Chem Biol Drug Des. 2013b;82:351–60.
Hassan GS, El-Sherbeny MA, El-Ashmawy MB, Bayomi SM, Maarouf AR, Badria FA. Synthesis and antitumor testing of certain new fused triazolopyrimidine and triazoloquinazoline derivatives. Arab J Chem. 2017;10:S1345–55.
Bera H, Chui WK, Gupta SD. Synthesis, in vitro evaluation of thymidine phosphorylase inhibitory activity, and in silico study of 1,3,5-triazin-2,4-dione and its fused analogues. Med Chem Res. 2013a;22:6010–21.
Dhall E, Jain S, Mishra A, Dwivedi J, Sharma S. Synthesis and evaluation of some phenyl substituted azetidine containing 1,2,4-triazole derivatives as antibacterial agents. J Heterocyl Chem. 2018;55:2859–68.
Sahin D, Bayrak H, Demirbas A, Alpaykaraoglu S. Design and synthesis of some azole derivatives as potential antimicrobial agents. Med Chem Res. 2012;21:4485–98.
Vatmurge NS, Hazra BG, Pore VS, Shirazi F, Chavan PS, Deshpande MV. Synthesis and antimicrobial activity of b-lactam-bile acid conjugates linked via triazole. Bioorg Med Chem Lett. 2008;18:2043–7.
Chernyshev VM, Pyatakov DA, Sokolov AN, Astakhov AV, Gladkov ES, Shishkina SV, et al. Partially hydrogenated 2-amino[1,2,4]triazolo[1,5-a]pyrimidines as synthons for the preparation of polycondensed heterocycles: reaction with chlorocarboxylic acid chlorides. Tetrahedron. 2014;70:684–701.
Abu-Hashem AA, Gouda MA. Synthesis and antimicrobial activity of some novel quinoline, chromene, pyrazole derivatives bearing triazolopyrimidine moiety. J Heterocyl Chem. 2017;54:850.
Tokala R, Bale S, Janrao IP, Vennela A, Kumar NP, Senwar KR, et al. (2018) Synthesis of 1,2,4-triazole-linked urea/thiourea conjugates as cytotoxic and apoptosis inducing agents. Bioorg Med Chem Lett. 2018;28:1919–24
Wang XF, Zhang S, Li BL, Zhao JJ, Liu YM, Zhang RL, et al. Synthesis and biological evaluation of disulfifides bearing 1,2,4-triazole moiety as antiproliferative agents. Med Chem Res. 2017;26:3367–74.
Branowska D, Ławecka J, Sobiczewski M, Karczmarzyk Z, Wysocki W, Wolin´ska E, et al. Synthesis of unsymmetrical disulfanes bearing 1,2,4-triazine scaffold and their in vitro screening towards anti-breast cancer activity. Monatsh Chem. 2018;149:1409–20.
Cincinelli R, Musso L, Artali R, Guglielmi M, Bianchino E, Cardile F, et al. Camptothecin-psammaplin A hybrids as topoisomerase I and HDAC dual-action inhibitors. Eur J Med Chem. 2018;143:2005–14.
Zhu SJ, Ying HZ, Wu Y, Qiu N, Liu T, Yang B, et al. Design, synthesis and biological evaluation of novel podophyllotoxin derivatives bearing 4β-disulfide/trisulfide bond as cytotoxic agents. RSC Adv. 2015;5:103172–83.
Liu HY, Wang HX, Li X, Wu Z, Li CW, Liu YM, et al. Synthesis, antitumor and antimicrobial evaluation of novel 1,3,4-thiadiazole derivatives bearing disulfide bond. Med Chem Res. 2018;27:1929–40.
Blanchard S, Bill E, Weyhermuller T, Wieghardt K. N,N-coordinated π radical anions of S-methyl-1-phenyl-isothiosemicarbazide in two five-coordinate ferric Complexes [FeIII(LMe·)2X] (X = CH3S−, Cl−). Inorg Chem. 2004;43:2324–9.
Acknowledgements
This study was financial supported by Tianjin Municipal Natural Science Foundation (18JCYBJC94900) and Training Project of Innovation Team of Colleges and Universities in Tianjin (TD13-5020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, Z., Li, X., Chi, CL. et al. Synthesis and antitumor effects of a new class of 1,2,4-triazole derivatives. Med Chem Res 30, 142–151 (2021). https://doi.org/10.1007/s00044-020-02652-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02652-y