Abstract
Conflict monitoring theory (CMT; Botvinick, Braver, Barch, Carter, & Cohen Psychological Review, 108, 624–652, 2001) states that response conflict, the simultaneous activation of two competing responses, increases task focus and reduces interference from irrelevant information. CMT also defines errors as conflict, and reduced interference effects have consistently been reported following errors (Ridderinkhof Psychological Research, 66, 312–323, 2002). However, previous computations of this posterror reduction of interference (PERI) have overlooked the congruency of the previous trial. This is problematic, because most errors are made on incongruent trials, creating a confound between (previous) accuracy and (previous) congruency. Therefore, it is likely that reduced interference following errors is in fact the congruency sequence effect (i.e., reduced interference following incongruent, relative to congruent, trials). Our results corroborate this idea by demonstrating that participants indeed showed significant PERI following a congruent trial, but inverse PERI following an incongruent trial. These findings are discussed in light of the adaptation-by-binding account (Verguts & Notebaert Psychological Review, 115, 518–525, 2008, Trends in Cognitive Sciences, 13, 252–257, 2009).
Similar content being viewed by others
Although we are continuously exposed to irrelevant and conflicting signals, we often succeed in selecting the signals that are relevant, while ignoring what is irrelevant. This quality is defined as cognitive control. In the laboratory, cognitive control is studied using conflict tasks. For example, in a Simon task, participants have to respond to the color of stimuli that are being presented on the left or the right side of the screen. Incongruent stimuli (the response location and the [irrelevant] stimulus location differ) are typically found to result in slower and more error-prone responses than do congruent stimuli (Lu & Proctor, 1995; Simon, 1969).
Interestingly, this interference effect (i.e., the difference between incongruent and congruent trials) is often reduced following incongruent trials, relative to congruent trials (the congruency sequence effect; Frith & Done, 1986; Gratton, Coles, & Donchin, 1992). This congruency sequence effect, according to the influential conflict monitoring theory (CMT; Botvinick, Braver, Barch, Carter, & Cohen, 2001), demonstrates that response conflict triggers an adaptive mechanism that enhances task-specific processes, leading to so-called conflict adaptation. Response conflict is defined by, and equated with, the simultaneous activation of two competing response units. On incongruent trials, one response is activated by the relevant dimension, and another by the irrelevant dimension (Cohen, Dunbar, & McClelland, 1990; Kornblum, 1994). However, the idea that the congruence sequence effect is a measure of conflict adaptation needs some nuance, since this is often confounded with feature repetition effects (Hommel, 2004; Mayr & Awh, 2009; Mayr, Awh, & Laurey, 2003; but see Duthoo & Notebaert, 2012) or contingency learning (Mordkoff, 2012; Schmidt & De Houwer, 2011).
Importantly, CMT also defines errors as conflicts, assuming that both the incorrect (executed) and correct response received activation. Consequently, CMT predicts reduced interference effects following errors. Indeed, posterror reduction of interference (PERI; King, Korb, von Cramon, & Ullsperger, 2010; Ridderinkhof, 2002; Ridderinkhof et al., 2002) has been observed. Other studies have failed to replicate this effect (Carp & Compton, 2009; Orr, Carp, & Weissman, 2012), or have even found increased interference following errors (Bombeke, Schouppe, Duthoo, & Notebaert, 2013). Surprisingly, none of these studies included Preceding Congruency as a factor in their analyses, even though the vast majority of errors in congruency tasks are made on incongruent trials (Hajcak, McDonald, & Simons, 2003; King et al., 2010). Therefore, what has been reported as PERI might have been confounded by the high proportions of congruent correct and incongruent incorrect trials. In order to show increased task focus following errors, we would need to account for previous congruency and demonstrate a smaller congruency effect following errors, regardless of the previous congruency (i.e., PERI after errors on both congruent and incongruent trials).
We designed two experiments that would allow us to include the factors Previous Congruency and Previous Accuracy. Experiment 1 was based on the study by Ridderinkhof (2002) in which PERI was first reported. In Experiment 2, we balanced the proportions of congruent and incongruent trials.
Experiment 1
In a paradigm based on Ridderinkhof (2002), we used a Simon task with .75/.25 probabilities for congruent/incongruent trials. A feedback mechanism encouraged participants to respond quickly while keeping accuracy above 85 %. To ensure reliable numbers of errors on both congruent and incongruent trials, 2,000 trials were administered.
Method
Participants
Twenty students at Ghent University (16 female, four male) participated (mean age = 18.7 years, SD = 1.6 years) for course credits.
Stimuli and material
Stimuli were presented on a 17-in. computer screen. The viewing distance was about 60 cm. A centrally presented black square contour (0.5 × 0.5 cm) was horizontally flanked by two larger black square contours (3.0 × 3.0 cm), with a center-to-center distance of 2.3 cm between the middle square and the lateral squares. The stimulus was a black or a white diamond (1.6 × 1.6 cm) presented in one of the lateral squares. Feedback (“0,” “5,” or “9”) was presented in the middle square (0.3 cm vertically and 0.2 cm horizontally). All of the stimuli were presented against a light-gray background, and responses were recorded using a Cedrus response box. The experiment was conducted using Tscope software (Stevens, Lammertyn, Verbruggen, & Vandierendonck, 2006).
Procedure
Participants had to respond to the color of the diamond by pressing, for instance, a left key when a black diamond or a right key when a white diamond was presented (counterbalanced between subjects). The participants were informed that on 75 % of the trials the location would correspond to the correct response side. However, it was stressed that the response should be based on the color of the figure. Each trial started with the presentation of a stimulus inside one of the lateral squares, until a response was given or 2 s had passed. Following response, feedback was presented for 750 ms, after which a new trial started. Participants earned points for performing quickly and accurately. A “0” was presented when the participant responded incorrectly, and a “5” when the response was correct. When the participant was correct and faster than his or her running average reaction time, a “9” was presented. The average was updated on every trial for congruent and incongruent trials separately. An updated score was presented after each block.
After the instructions, participants performed a practice block of 32 trials. Next, they received instructions about the feedback procedure and were told that the participant with the most points would win an additional reward of €10. The experiment consisted of 20 blocks of 100 trials.
Results
Responses faster than 100 ms or exceeding the response deadline, as well as their preceding and subsequent trials, were excluded from the analysis. Postcorrect trials that were followed by a correct response were also discarded. On average, 276 trials (SD = 130) were included in the analysis. The mean reaction time was 388 ms (SD = 47 ms), and the mean error rate was 7 % (SD = 4 %).
Both error rates and reaction times were first analyzed with only Previous Accuracy and Current Congruency as fixed factors. Second, we included the factor Previous Congruency, as well as Stimulus Sequence (color repetition or alternation from trial n – 1 to n; see also Braem, Verguts, & Notebaert, 2011) to measure the effects of low-level stimulus repetitions on response repetition effects. Importantly, although the factor Stimulus Sequence allowed us to have an idea of the relative contribution of low-level repetitions, it did not rule out feature repetition effects, since the sequence of the irrelevant feature could not be accounted for (Hommel, 2004; Mayr & Awh, 2009; Mayr et al., 2003).
The results were analyzed using linear mixed-effects models, as implemented in the R package lme4 (Bates, Maechler, Bolker, & Walker, 2013). As was proposed by Barr, Levy, Scheepers, and Tily (2013), we used a maximal linear mixed-effects model with a random effect for subjects. However, when we included the factors Previous Congruency and Stimulus Sequence, the model did not converge. We therefore simplified the random-effects structure by removing the random slope for previous congruency.
Error rates were analyzed using a logistic link function. For reaction times—a continuous variable—we report F statistics with Kenward–Roger adjustment of the degrees of freedom (Kenward & Roger, 1997). For binary responses, no such small-sample adjustments of the degrees of freedom have been proposed in the literatureFootnote 1; therefore, we adopted the standard strategy of reporting χ 2 statistics.Footnote 2
PERI is calculated by subtracting the congruency effect (incongruent – congruent) following an error from the congruency effect following a correct response. Because we selected trials present in specific local sequences (i.e., C–X–E for postcorrect trials and E–X for posterror trials), all trials originated from moments close to each other in time, rendering this analysis immune to global performance fluctuations (Dutilh et al., 2012). However, the significance of our results did not differ when we used all postcorrect trials.
Error rates
The traditional analysis for error rates (only previous accuracy and current congruency were included) showed a main effect of current congruency, χ 2(1) = 282.89, p < .001, but no significant effect of previous accuracy, χ 2(1) < 1, p = .37. We observed a significant interaction of congruency and previous accuracy, χ 2(1) = 21.63, p < .001, showing that the congruency effect after an error (15 %) was smaller than that effect on trials following a correct response (23 %).
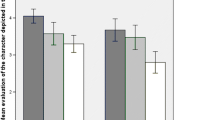
Extending the analysis with the factors Previous Congruency and Stimulus Sequence again showed a main effect of current congruency, χ 2(1) = 126.60, p < .001, indicating that participants made fewer errors on congruent (3 %) than on incongruent (17 %) trials. No significant effects were apparent of previous congruency, χ 2(1) < 1, p = .50, or previous accuracy, χ 2(1) = 1.53, p = .225. The congruency of the previous trial interacted significantly with the congruency of the current trial (16 %), χ 2(1) = 52.57, p < .001. However, we found no significant interaction of previous accuracy with previous congruency, χ 2(1) < 1, p = .94, or with the congruency of the current trial, χ 2(1) < 1, p = .71. The three-way interaction was significant, χ 2(1) = 53.02, p < .001. This interaction is shown in Fig. 1a. Significant PERI occurred after a congruent trial (18 %), χ 2(1) = 30.98, p < .001, but after an incongruent trial, significant inverse PERI emerged instead (–15 %), χ 2(1) = 21.77, p < .001. This pattern of results did not interact significantly with stimulus sequence, χ 2(1) = 1.61, p = .20.
a Error rates (as percentages) and b reaction times (in milliseconds) dependent on previous accuracy and current congruency, following congruent and incongruent trials. Error bars represent 95 % confidence intervals around the means. For each level of previous accuracy, the percentages of previous congruent and incongruent trials can be found in the graphs
Reaction times
Analyzing reaction times with only Previous Accuracy and Current Congruency as factors showed main effects of both current congruency, F(1, 18.3) = 118.25, p < .001, and previous accuracy, F(1, 22.1) = 52.37, p < .001. However, we found no significant interaction of previous accuracy and current congruency in this data set, F(1, 4987.2) < 1, p = .57.
When we included the factors Previous Congruency and Stimulus Sequence, a significant congruency effect was visible (55 ms), F(1, 19.4) = 6.99, p < .05, as well as a significant effect of previous accuracy, F(1, 23.8) = 42.64, p < .001, showing posterror slowing (63 ms). The effect of previous congruency was not significant, F(1, 4935.9) = 2.15, p = .14. The congruency of the current trial showed a significant interaction with previous congruency, F(1, 4943.1) = 15.32, p < .001, indicating a congruency sequence effect (35 ms). We also observed a significant interaction of previous accuracy and previous congruency, F(1, 4937.1) = 4.22, p < .05, showing smaller posterror slowing following incongruent trials (54 ms) than following congruent trials (73 ms). Previous accuracy also interacted significantly with the congruency of the current trial, F(1, 4967.4) = 6.55, p < .05 (–22 ms, inverse PERI). Interestingly, the three-way interaction of previous congruency, previous accuracy, and current congruency was also significant, F(1, 4944.6) = 18.15, p < .001. This interaction is shown in Fig. 1b. For postcongruent trials, we found no significant PERI (16 ms), F(1, 2592.43) = 2.03, p = .15. After an incongruent trial, however, significant inverse PERI was apparent (–60 ms), F(1, 2109.81) = 22.25, p < .001. This pattern of results did not interact significantly with stimulus sequence, F(1, 4953.2) < 1, p = .58.
Discussion
In line with the results of Ridderinkhof (2002), Experiment 1 showed significant PERI in error rates when the factor Previous Congruency was omitted. This effect was not replicated in reaction times. However, when previous congruency was included, a significant three-way interaction showed significant PERI following a congruent trial, but inverse PERI following an incongruent trial.
Experiment 2
The method of this experiment was identical to that of Experiment 1, with the exception that half of the trials were congruent and the other half incongruent. This balanced design served as a replication, while simultaneously controlling for contingency learning by no longer allowing participants to predict the response on the basis of the irrelevant information (Mordkoff, 2012; Schmidt & De Houwer, 2011).
Twenty students at Ghent University (17 female, three male) participated (mean age = 19.3 years, SD = 1.8 years) for course credits. On average, 378 trials (SD = 154) were included in the analysis. The mean reaction time was 364 ms (SD = 38 ms), and the mean error rate was 11 % (SD = 6 %).
Results
Error rates
Analyzing error rates with only Previous Accuracy and Current Congruency as factors showed a main effect of current congruency, χ 2(1) = 45.79, p < .001, but no significant effect of previous accuracy, χ 2(1) < 1, p = .75. We observed a significant interaction of congruency and previous accuracy, χ 2(1) = 5.76, p = .02, showing that the congruency effect after an error (7 %) was smaller than that effect after a correct response (11 %).
Extending the analysis with the factors Previous Congruency and Stimulus Sequence again showed a main effect of current congruency, χ 2(1) = 38.84, p < .001, indicating that participants made fewer errors on congruent (5 %) than on incongruent (13 %) trials. No significant effects were apparent of previous accuracy, χ 2(1) = 1.10, p = .30, or previous congruency, χ 2(1) < 1, p = .63. The congruency of the previous trial interacted significantly with the congruency of the current trial (11 %), χ 2(1) = 67.23, p < .001. We found no significant interaction of previous accuracy and previous congruency, χ 2(1) = 1.12, p = .29, but the interaction of previous accuracy and the congruency of the current trial was marginally significant, χ 2(1) = 3.65 1, p = .06, showing a smaller congruency effect following an error (6 %) than following a correct response (8 %). The three-way interaction of previous accuracy, previous congruency, and current congruency was significant, χ 2(1) = 123.18, p < .001. This interaction is shown in Fig. 2a. After a congruent trial, significant PERI emerged (20 %), χ 2(1) = 64.34, p < .001, but after an incongruent trial, significant inverse PERI was seen instead (–12 %), χ 2(1) = 48.40, p < .001. This pattern of results did not interact significantly with stimulus sequence, χ 2(1) = 1.11, p = .28.
a Error rates (as percentages) and b reaction times (in milliseconds) dependent on previous accuracy and current congruency, following congruent and incongruent trials. Error bars represent 95 % confidence intervals around the means. For each level of previous accuracy, the percentages of previous congruent and incongruent trials can be found in the graphs
Reaction times
Analyzing reaction times with only previous accuracy and current congruency revealed a main effect of previous accuracy, F(1, 19) = 4.64, p < .05, but not an effect of current congruency, F(1, 19) < 1, p = .46. No significant interaction of previous accuracy and current congruency was apparent, F(1, 6542.9) < 1, p = .96.
When we included the factors Previous Congruency and Stimulus Sequence, a significant congruency effect emerged (27 ms), F(1, 20.8) = 54.41, p < .001, as well as a significant effect of previous accuracy, F(1, 19.4) = 34.19, p < .001, showing posterror slowing (56 ms). The effect of previous congruency was not significant, F(1, 6538.4) < 1, p = .61. The congruency of the current trial showed a significant interaction with previous congruency (35 ms), F(1, 6501.3) = 39.67, p < .001, showing a congruency sequence effect. Previous accuracy did not interact significantly with previous congruency, F(1, 6536.4) = 2.36, p = .12, or with current congruency, F(1, 6534.7) = 1.38, p = .24. The three-way interaction of previous accuracy, previous congruency, and current congruency was significant, F(1, 6501.8) = 40.53, p < .001. This interaction is shown in Fig. 2b. After a congruent trial, significant PERI was apparent (31 ms), F(1, 2858.59) = 13.40, p < .001, but after an incongruent trial, we observed significant inverse PERI instead (–45 ms), F(1, 3620.8) = 30.12, p < .001. Importantly, this effect did not interact significantly with stimulus sequence, F(1, 6535.9) = 1.41, p = .24.
General discussion
In order to better understand behavioral adjustments following errors, we investigated the modulation of posterror reduction of interference following congruent and incongruent trials separately. Omitting the factor Previous Congruency, in line with Ridderinkhof (2002), resulted in significant PERI in the error rates from both experiments. However, when previous congruency was included, PERI was observed following congruent trials, but inverse PERI following incongruent trials, casting doubt on earlier reports of the PERI effect (King et al., 2010; Ridderinkhof, 2002; Ridderinkhof et al., 2002).
Our results thus pose a challenge for CMT. Following congruent trials, the expected pattern was observed. However, following incongruent trials, significant inverse PERI effects were demonstrated for both error rates and reaction times. Regardless of whether errors on congruent or incongruent trials elicit more conflict (Yeung, Botvinick, & Cohen, 2004), error trials should elicit more conflict than correct trials do, and CMT would always predict PERI. One could assume that making an error on an incongruent trial induces roughly the same amount of conflict as does a correct incongruent trial, but this would still not be compatible with the finding of inverse PERI after incongruent trials. This pattern of results could, however, be described in terms of the adaptation-by-binding account (Verguts & Notebaert, 2008, 2009). This account proposes that conflict engages Hebbian learning processes on all currently active representations. This mechanism results in a stronger task focus following conflict trials, and a weaker task focus following no-conflict trials. Interestingly, both the up- and down-regulation of cognitive control occur only on correct trials, because the Hebbian mechanism requires correctly activated associations. It is rather speculative to describe the activation pattern on errors; therefore, the safest assumption is that no adjustments occur following errors. However, as compared to postcorrect trials, posterror trials would show a smaller congruency effect for congruent trials (i.e., PERI) and a larger congruency effect for incongruent trials (i.e., reversed PERI), due to the adaptation following correct trials.
Contrary to our findings, Maier, Yeung, and Steinhauser (2011) calculated PERI following incongruent trials only and showed a reduction of interference following flanker errors (responding to the irrelevant flanker feature), relative to correct trials or nonflanker errors (responses to neither the target nor the flanker). However, in their design, neutral trials were presented instead of congruent trials. Therefore, focusing on the task-irrelevant dimension was never beneficial for efficient task performance, promoting a task strategy that probably differed from the one in our paradigm. Still, further research will be needed to see under which conditions errors might indeed help conflict processing.
Our data also revealed posterror slowing (Rabbitt & Rodgers, 1977), which has traditionally been described as the result of an increase in cognitive control, and as such is predicted to be observed alongside a posterror accuracy increase (Botvinick et al., 2001). The lack of accuracy improvements after errors in the literature (Bombeke et al., 2013; Hajcak et al., 2003; King et al., 2010; Ridderinkhof, 2002) has given rise to so-called nonfunctional explanations for posterror slowing (Jentzsch & Dudschig, 2009; Notebaert et al., 2009). Our data show an interesting dissociation between posterror slowing and PERI: Although PERI was only observed following congruent trials, posterror slowing was observed following congruent and incongruent trials. This dissociation is again an indication that the originally reported PERI effects reflected a modulation by congruency, primarily driven by congruent trials (Compton, Huber, Levinson, & Zheutlin, 2012) rather than by errors.
Notes
For binary dependent variables, small-sample inference is approximate because the number of possible outcomes is limited.
χ 2 and F statistics are related in the same way as z and t statistics: F and t assume a finite sample, and with increasing sample size they converge with the χ 2 and z statistics, which assume an infinite sample.
References
Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68, 255–278. doi:10.1016/j.jml.2012.11.001
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2013). lme4: Linear mixed-effects models using Eigen and S4 (R package version 1.0-5). Retrieved from http://cran.r-project.org/package=lme4
Bombeke, K., Schouppe, N., Duthoo, W., & Notebaert, W. (2013). The effect of alcohol and placebo on post-error adjustments. Frontiers in Human Neuroscience, 7, 3. doi:10.3389/fnhum.2013.00003
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108, 624–652. doi:10.1037/0033-295X.108.3.624
Braem, S., Verguts, T., & Notebaert, W. (2011). Conflict adaptation by means of associative learning. Journal of Experimental Psychology: Human Perception and Performance, 37, 1662–1666.
Carp, J., & Compton, R. J. (2009). Alpha power is influenced by performance errors. Psychophysiology, 46, 336–343.
Cohen, J. D., Dunbar, K., & McClelland, J. L. (1990). On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review, 97, 332–361. doi:10.1037/0033-295X.97.3.332
Compton, R. J., Huber, E., Levinson, A. R., & Zheutlin, A. (2012). Is “conflict adaptation” driven by conflict? Behavioral and EEG evidence for the underappreciated role of congruent trials. Psychophysiology, 49, 583–589.
Duthoo, W., & Notebaert, W. (2012). Conflict adaptation: It is not what you expect. Quarterly Journal of Experimental Psychology, 65, 1993–2007. doi:10.1080/17470218.2012.676655
Dutilh, G., van Ravenzwaaij, D., Nieuwenhuis, S., van der Maas, H. L. J., Forstmann, B. U., & Wagenmakers, E.-J. (2012). How to measure post-error slowing: A confound and a simple solution. Journal of Mathematical Psychology, 56, 208–216. doi:10.1016/j.jmp.2012.04.001
Frith, C. D., & Done, D. J. (1986). Routes to action in reaction time tasks. Psychological Research, 48, 169–177.
Gratton, G., Coles, M. G. H., & Donchin, E. (1992). Optimizing the use of information: Strategic control of activation of responses. Journal of Experimental Psychology: General, 121, 480–506. doi:10.1037/0096-3445.121.4.480
Hajcak, G., McDonald, N., & Simons, R. F. (2003). To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40, 895–903. doi:10.1111/1469-8986.00107
Hommel, B. (2004). Event files: Feature binding in and across perception and action. Trends in Cognitive Sciences, 8, 494–500. doi:10.1016/j.tics.2004.08.007
Jentzsch, I., & Dudschig, C. (2009). Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Quarterly Journal of Experimental Psychology, 62, 209–218.
Kenward, M. G., & Roger, J. H. (1997). Small sample inference for fixed effects from restricted maximum likelihood. Biometrics, 53, 983–997. doi:10.2307/2533558
King, J. A., Korb, F. M., von Cramon, D. Y., & Ullsperger, M. (2010). Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. Journal of Neuroscience, 30, 12759–12769.
Kornblum, S. (1994). The way irrelevant dimensions are processed depends on what they overlap with: The case of Stroop-and Simon-like stimuli. Psychological Research, 56, 130–135.
Lu, C.-H., & Proctor, R. W. (1995). The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychonomic Bulletin & Review, 2, 174–207. doi:10.3758/BF03210959
Maier, M. E., Yeung, N., & Steinhauser, M. (2011). Error-related brain activity and adjustments of selective attention following errors. NeuroImage, 56, 2339–2347.
Mayr, U., & Awh, E. (2009). The elusive link between conflict and conflict adaptation. Psychological Research, 73, 794–802. doi:10.1007/s00426-008-0191-1
Mayr, U., Awh, E., & Laurey, P. (2003). Conflict adaptation effects in the absence of executive control. Nature Neuroscience, 6, 450–452.
Mordkoff, J. T. (2012). Three reasons to avoid having half of the trials be congruent in a four-alternative forced-choice experiment on sequential modulation. Psychonomic Bulletin & Review, 19, 750–757. doi:10.3758/s13423-012-0257-3
Notebaert, W., Houtman, F., Van Opstal, F., Gevers, W., Fias, W., & Verguts, T. (2009). Post-error slowing: An orienting account. Cognition, 111, 275–279. doi:10.1016/j.cognition.2009.02.002
Orr, J. M., Carp, J., & Weissman, D. H. (2012). The influence of response conflict on voluntary task switching: A novel test of the conflict monitoring model. Psychological Research, 76, 60–73.
Rabbitt, P., & Rodgers, B. (1977). What does a man do after he makes an error? An analysis of response programming. Quarterly Journal of Experimental Psychology, 29, 727–743. doi:10.1080/14640747708400645
Ridderinkhof, K. R. (2002). Micro- and macro-adjustments of task set: Activation and suppression in conflict tasks. Psychological Research, 66, 312–323. doi:10.1007/s00426-002-0104-7
Ridderinkhof, K. R., de Vlugt, Y., Bramlage, A., Spaan, M., Elton, M., Snel, J., & Band, G. P. H. (2002). Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science, 298, 2209–2211.
Schmidt, J. R., & De Houwer, J. (2011). Now you see it, now you don’t: Controlling for contingencies and stimulus repetitions eliminates the Gratton effect. Acta Psychologica, 138, 176–186. doi:10.1016/j.actpsy.2011.06.002
Simon, J. R. (1969). Reactions toward the source of stimulation. Journal of Experimental Psychology, 81, 174–176. doi:10.1037/h0027448
Stevens, M., Lammertyn, J., Verbruggen, F., & Vandierendonck, A. (2006). Tscope: A C library for programming cognitive experiments on the MS windows platform. Behavior Research Methods, 38, 280–286. doi:10.3758/BF03192779
Verguts, T., & Notebaert, W. (2008). Hebbian learning of cognitive control: Dealing with specific and nonspecific adaptation. Psychological Review, 115, 518–525. doi:10.1037/0033-295X.115.2.518
Verguts, T., & Notebaert, W. (2009). Adaptation by binding: A learning account of cognitive control. Trends in Cognitive Sciences, 13, 252–257.
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111, 931–959. doi:10.1037/0033-295X.111.4.931
Author note
The work of L.V.d.B. and W.N. is supported by FWO-Vlaanderen Grant No. B/11792/02, and the work of S.B. is supported by FWO-Vlaanderen Grant No. G.0098.09N. The contribution of W.N. is supported by the Ghent University Special Research Fund (Grant No. B/09928/02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van der Borght, L., Braem, S. & Notebaert, W. Disentangling posterror and postconflict reduction of interference. Psychon Bull Rev 21, 1530–1536 (2014). https://doi.org/10.3758/s13423-014-0628-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-014-0628-z