Abstract
We investigated the ability of North American river otters (Lontra canadensis) to visually discriminate between 2D objects. The otters learned to discriminate between stimuli using multiple visual features and then were tested with stimuli in which one of the features was eliminated (color or shape). Two adult otters were trained in a two-alternative forced choice task to discriminate between a red circle and a blue triangle. Test sessions included probe trials containing novel shapes, colors, or shape-color combinations. Both otters successfully learned to discriminate between stimuli varying in multiple features. One of the otters was able to successfully discriminate between novel test stimuli when either color or shape were eliminated as salient features. This study was the first to explore the ability of L. canadensis to use different visual features to recognize objects and provides some preliminary evidence for color vision in this species. This research adds to the sparse literature on perceptual and cognitive capabilities in otters and can be used to support future conservation efforts for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
North American river otters (Lontra canadensis) belong to the subfamily Lutrinae within the family Mustelidae. There are 13 extant otter species that vary in terms of size, diet, habitat, sociality, group size, and foraging habits (Yoxon & Yoxon, 2014). Little research has been conducted on cognitive and perceptual abilities in otter species, with only a single behavioral study on visual object perception in North American river otters (Slack, 1966). Recent studies on Asian small-clawed otters (Aonyx cinereus) and giant otters (Pteronura brasiliensis) show that they can participate in behavioral research exploring cognitive and perceptual capabilities (Frick, Friedman, Peranteau, Beacham, & Kuczaj, 2016; Perdue, Snyder, & Maple, 2013; Schmelz, Duguid, Bohn, & Volter, 2017; Svoke, Snyder, & Elgart, 2014). The variation in behavior and ecology amongst the genetically-related otter species presents an opportunity to explore how these variables are related to their sensory perception and cognitive skills (Gormley, 2015). The purpose of the current study was to investigate visual object recognition in North American river otters with the goal of determining which visual features they use to discriminate among objects.
Understanding the life history of river otters is important when considering how they may use visual perception to identify objects or other animals. North American river otters live along the Atlantic and Pacific coasts of higher latitudes of North America as well as in the northern interior of the continent in Canada and almost all of the USA except the arid southwest (Johnson, 2000; Larivière & Walton, 1998). This widespread species inhabits rivers, marshes, estuaries, and coastal environments (Gallant, Vasseur, Dumond, Tremblay, & Bérubé, 2009; Guertin, Harestad, & Elliott, 2009; Johnson, 2000; Larivière & Walton, 1998; Scordino, Gearin, Riemer, & Iwamoto, 2016). North American river otters are considered to be a more gregarious species than other otters (Kruuk, 2006). Their social groups are composed of families (females and their young, sometimes with additional adult otters) or unrelated males (Kruuk, 2006). Male river otters form groups based on familiarity and past experience, rather than kinship (Hansen, McDonald, Groves, Maier, & Ben-David, 2009). River otters have overlapping home ranges, suggesting that they are typically social rather than territorial or solitary (Gorman, Erb, McMillan, & Martin, 2006). Conspecific recognition likely involves multiple sensory modalities, including vision (Kruuk, 2006).
Across all studied environments and populations, river otters’ primary food source seems to be fish, followed by crustaceans and insects, with opportunistic tendencies to infrequently pursue widely varied food sources including birds, mammals, reptiles, and amphibians (Crimmins, Roberts, & Hamilton, 2009; Fretueg, Martin, Widga, & Ruez, 2015; Guertin et al., 2009; Larsen, 1984; Penland & Black, 2009; Reid, Code, Reid, & Herrero, 1994; Scordino et al., 2016; Serfass, M. Rymon, & Brooks, 1990; Toweill, 1974). Otters seem to have preferences related to the size and speed of their prey (Crimmins et al., 2009), which likely indicates a preference for food that will yield the greatest metabolic payoff for the energy expended while foraging. Food consumption seems to be controlled by both dietary preferences and abundance of food sources depending on location and season (Crowley et al., 2013; Scordino et al., 2016). The ability to perceive visual features like shape and color may contribute to their ability to select preferred prey items. Green (1977) found that it takes European river otters (L. lutra) four times longer to find prey in turbid waters compared to clear water. The time necessary to find prey with or without the use of vibrissae is the same in clear water, but without vibrissae otters take 20 times longer in turbid water than in clear water. Green’s study (1977) suggests that river otters forage predominantly using vision, supplementing with tactile perception when visual conditions are suboptimal. North American river otters may use vision to identify predators, prey, and conspecifics.
Limited research has been done on visual acuity in only two species of otters, with no studies focused on L. canadensis. The visual acuity of the sea otter is approximately the same as some pinnipeds, whereas visual acuity in the Asian small-clawed otter is similar to the mink tested in air (Sinclair, Dunstone, & Poole, 1974). Visual acuity is commonly measured as minutes of arc, where a lower number constitutes higher acuity (humans with 20/20 vision can resolve a spatial pattern separated by a visual angle of 1 min of arc). In an anatomical study of retinal resolution, sea otters were found to have underwater visual acuity of 7 min of arc (Mass & Supin, 2000). Two Asian small-clawed otters (Amblonyx cineria) had equivalent aerial and underwater visual acuity in bright light conditions (Balliet & Schusterman, 1971). The visual acuity of these Asian small-clawed otters (15–16 min of arc in water, 14–15 min of arc in air) was worse than the underwater visual acuity of harbor seals (8 min of arc), stellar sea lions (8 min of arc), and California sea lions (6 min of arc in water and air; Balliet & Schusterman, 1971; Fobes & Smock, 1981). In dim light conditions, Asian small-clawed otters show superior visual acuity in air (38–39 min of arc) compared to underwater (57–58 min of arc; Schusterman & Barrett, 1973). A sea otter (Enhydra lutris) trained to select the larger of two disks presented underwater showed inferior performance compared to California sea lions (Gentry & Peterson, 1967). The conclusion we can draw from the above studies is that the otter eye appears to be emmetropic in air with specialized adaptations for underwater vision. This is in contrast to pinnipeds, whose eyes are emmetropic underwater with dioptric mechanisms for seeing in air (Schusterman & Barrett, 1973). Human vision is emmetropic in air, and superior to otters when tested in air (0.7 min of arc; Shlaer, 1937) but inferior underwater (Schusterman & Balliet, 1970).
Behavioral and anatomical studies conducted with three species of otters suggests that they have dichromatic color vision. Non-primate mammals are typically dichromats (Jacobs, 1993). Dichromatic vision in diurnal terrestrial mammals usually involves one cone that is green to red sensitive (L cone) and one cone that is blue to ultraviolet sensitive (S cone; Griebel & Peichel, 2003). Sea otters (Enhydra lutris) and European river otters (Lutra lutra) possess only two cones types: S cones and M/L cones (Levenson et al., 2006; Peichl, Behrmann, & Kröger, 2001). European river otters (Lutra lutra) could discriminate blue and green from various shades of gray in a behavioral study (Kasprzyk, 1990, cited in Griebel & Peichel, 2003). The Asian small-clawed otter (A. cineria) discriminated between gray and red or green (Balliet, 1970). In a recent study, A. cineria showed the ability to discriminate gray from white, green, blue, and red (Svoke, Snyder, & Elgart, 2014). In Svoke et al.’s (2014) study, one otter could discriminate blue versus green and blue versus red, but not red versus green. Svoke et al. (2014) suggested that the otter’s failure to discriminate red versus green may have been an inability to perceive a difference between the colors or a methodological issue (e.g., the color cards for red and green may not have been at the ideal wavelengths for the peak sensitivity of the otters’ cones). Taken together, these studies suggest that otters can make color discriminations. Note that no studies have been conducted on color perception in L. canadensis.
Only one study on shape perception has been conducted with any species of otter. Two North American river otters were presented with a series of 11 stimulus pairs (Slack, 1966). The 2D stimuli were black figures on a white background, including simple geometric shapes, complex shapes, symbols, and letters (e.g., square, triangle, circle, cross, arrows, the letters E, V, S, F). In the two-alternative forced choice task, the otters had to meet a criterion before proceeding to the next stimulus pair. The male otter succeeded with 11 pairs whereas the female otter succeeded on two pairs. The number of trials to reach criterion varied with stimulus pair, showing that the male required the most trials to discriminate pairs 1 and 7 (black square vs. empty white background; S vs. #). The otter’s performance was influenced by an issue with the apparatus, motivation, and activities prior to test sessions. Slack (1966) did not explore which features of the stimuli the otters may have used to make the discriminations. Slack’s (1966) study provided preliminary evidence that otters can discriminate between 2D shapes.
Many non-human animals have demonstrated the ability to discriminate between 2D or 3D stimuli varying in shape, including jungle crows (Bogale & Sugita, 2013), parakeets (Colgan, Inman, & Gambrel, 1971), chickens (Zolman, 1969), pigeons (Young, Peissig, & Wasserman, 2001), opossum (James & MacFarland, 1966), rats (Minini & Jeffrey, 2006; Oldfield-Box, 1969), monkeys (Tanaka, 2000), honeybees (Srinivasan, 2010), white rhinos (Daniel & Mikulka, 1998), octopuses (Sutherland, 1959; Sutherland, 1969), sharks (Fuss, Bleckmann, & Schluessel, 2014; Fuss & Schluessel, 2015), dolphins (Harley, Roitblat, & Nachtigall, 1996; von Fersen, Schall, & Gunturken, 2000), fish (DeLong, Keller, Wilcox, Fobe, & Keenan, 2018; Siebeck, Litherland, & Wallis, 2009), and sea lions (Mauck & Dehnhardt, 1997; Schusterman & Thomas, 1966). Thus, the ability to discriminate shapes appears in birds, mammals, fish, and insects occupying a variety of habitats. Mustelid species other than L. canadensis appear to have been tested for shape discrimination ability in only a couple studies. New Zealand ferrets (Mustela putorius) were able to discriminate a solid upright triangle versus an inverted triangle and an upright triangle versus a cross (Pollard, Beale, Lysons, & Preston, 1967). The discriminations were maintained when the stimuli were modified to outlines or rotated (Pollard et al., 1967). Mink (Mustela vision) and ferrets (Mustela furo) achieved performance similar to primates in a learning-set formation task, although the stimuli varied in brightness, surface area, and thickness in addition to shape (Doty, Jones, & Doty, 1967).
The purpose of the current study was to investigate the ability of North American river otters to learn to discriminate among 2D stimuli. The first goal was to determine whether otters who learned to discriminate between objects with multiple features available as cues could continue to do so when a salient feature was eliminated. The second goal was to determine whether otters would show a preference for using either shape or color to discriminate between stimuli. Color perception in L. canadensis has never been tested and shape perception has not been well studied (Slack, 1966). No studies have been conducted comparing the ability to use different visual features for object recognition in any species of otter. Two subjects were trained in a two-alternative forced choice task to discriminate between two stimuli (a red circle and a blue triangle). Then, the otters were tested to examine whether they could discriminate between objects when shape was removed as a cue (e.g., red circle vs. blue circle) or color was removed as a cue (e.g., red circle vs. red triangle).
Color and shape were chosen as the salient features in this object discrimination task based on previous research. There is evidence that other otter species can make color discriminations, so we predicted that the North American river otters would also utilize color cues (Balliet, 1970; Kasprzyk, 1990, cited in Griebel & Peichel, 2003; Svoke, Snyder, & Elgart, 2014). Shape was selected as a feature since the North American river otters in Slack’s (1966) study were able to discriminate shapes (both otters successfully discriminated between a circle and triangle). The first hypothesis was that the otters would be able to use fewer features to discriminate between objects in the test after learning the discrimination with multiple features present. The second hypothesis was that the otters would show equivalent performance on shape probes (objects differing primarily in shape) and color probes (objects differing primarily in color) because no research to date with this species has tested color perception and only one study has examined shape perception (Slack, 1966), so there was no reason to predict superior performance using either color or shape cues.
Method
Subjects
The subjects were two North American river otters (one male, one female) housed at the Seneca Park Zoo in Rochester, NY, USA. The female, Heather, was 13 years of age and the male, Sailor, was 9 years of age at the start of the study in June 2016. Heather and Sailor were unrelated. Heather had resided at the Seneca Park Zoo since 2009, and Sailor had resided at the same zoo since 2012. No veterinary exams revealed any evidence of eye diseases, so it was assumed that both subjects had normal vision. Prior to the current study, Heather participated in training for a match-to-sample task for 3 years (2011–2013) but she never met the training criterion so she did not continue with the task. This match-to-sample task utilized a large variety of 3D stimuli, including the stimuli from the current study. Heather was also target-trained to approach her 3D positive stimulus in the current study (red circle) in 2010 (see pre-training section). Heather displayed this targeting behavior for an otter training demonstration for zoo visitors two to five times per week during June, July, and August from 2011 to 2017. Sailor was experimentally-naïve and did not participate in the summer training demonstrations.
The otter habitat in the Seneca Park Zoo contained two areas, one for public exhibition and an off-exhibit region. The public exhibition habitat (33.5 m ×18.3 m) was composed of a large upper pool (9.1 m ×2.4 m ×1.8 m) with a waterfall leading down to a lower pool (9.1 m ×4.6 m × 1.5 m) surrounded by dirt banks covered in grass and flowers, shrubbery, felled conifer trees, and solid and hollowed-out logs. This habitat also contained enrichment devices such as a tunnel made of hollow plastic tubing, plastic rafts, buoys, and a jellyfish float (trash can lid with mesh straps attached). Enrichment devices sometimes floated in the upper pool but were removed from the central pool area before sessions. The public exhibition habitat had an observation glass window divided into three panes (central window = 3.5 m in length, side windows = 1.7 m in length) through which guests could view both the underwater and land portion of the habitat. The off-exhibit habitat was composed of two cement-based areas surrounded completely by chain-linked fencing and covered with a metal roof. These enclosures were located outdoors. Each enclosure was divided into pens connected with gates that could be lifted by the keeper to move the animals from one area of the enclosure to another. Each enclosure contained dens (plastic dog houses) and enrichment devices such as plastic slides, hammocks, small plastic pools, and blankets. Heather and Sailor were housed together with access to three adjacent pens (each one was approximately 2 m ×1.5 m).
The otter’s daily diet consisted of approximately 230 g of fish [capelin (Mallotus villosus), herring (Clupea harengus), and/or mackerel (Scomber scombrus)] and one cup of dog chow mixed with 280 g of horse meat in the afternoon. Their regular diet was used as a primary food reinforcer. During training and testing otters were fed 75–100% of their regular morning diet of fish. The otters received their entire daily diet, regardless of performance during study sessions. The otters’ diet was determined by the animal care staff at the Seneca Park Zoo. The otters received the same diet over the course of the study in 2016 and 2017.
Stimuli
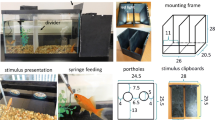
Two types of stimuli were used: 3D painted metal objects, and 2D shapes of the same dimensions printed in the center of white heavy matte paper squares (27.5 cm ×27.5 cm). The stimuli included a red circle (diameter = 22 cm, surface area = 380 cm2) and a blue equilateral triangle (height = 20.5 cm, sides = 24 cm, surface area = 246 cm2). The 3D stimuli had the same length and width as the 2D stimuli, but were 0.3 cm thick and included 11.4 cm ×2.5 cm handles on the back. The 3D stimuli are shown in Fig. 1. There were two exemplars of each of the 3D stimuli. The 3D stimuli were originally used by the zookeeper to train Heather to “target” (approach and touch the stimulus for a food reward). For both otters, only the 3D stimuli were used during the pre-training phase. For both otters, the 3D stimuli and the 2D stimuli were used during the training phase. In the test phase, only the 2D stimuli were used for both otters. A 5-cm loop of clear monofilament line was attached to all 2D stimuli with heavy-duty tape, and zip ties were fastened to hang them in the correct positions. Sailor’s 2D stimuli were laminated to prevent deterioration, since he could touch his stimuli (see procedure section).
In the pre-training and training phases, each otter viewed only one positive rewarded stimulus (S+) and one negative unrewarded stimulus (S-). Heather’s S+ was the red circle and her S- was the blue triangle. Sailor’s S+ was the blue triangle and his S- was the red circle. The stimuli used in the test phase included the training stimuli as well as additional test probes. There were two sets of test probes: the shape probes and the color probes. The shape probes tested the otters’ ability to discriminate between stimuli on the basis of shape (each stimulus pair was the same color). These three pairs included one pair the color of the otter’s training S+, one pair the color of the otter’s training S-, and one pair that was a novel color (black). The color probes tested the otters’ ability to discriminate stimuli on the basis of color (each stimulus pair was the same shape). These three pairs included one pair the same shape as the otter’s S+, one pair the same shape as the otter’s S-, and one pair that was a novel shape (hexagon). Table 1 shows all stimuli used in the test phase.
Experimental set-up
We used two different experimental set-ups: one for Heather and one for Sailor. This was done because Sailor was trained and tested under protected contact protocol and needed to remain within his enclosure while interacting with the trainer. The experimental set-up and procedure for each otter will be discussed separately. For all sessions with both otters, the same zookeeper served as the animal trainer throughout the study. Nine different people served as research assistants in the experimenter or recorder role.
A schematic of the experimental set-up we used for Heather is shown in Fig. 2. All materials were set up inside the ECO Center building against the central glass window. The ECO Center building houses the otter exhibit, as well as other animals (reptiles, fish), and serves as the inside viewing area for zoo visitors to observe the otters swimming in the pool. Two 5-cm plastic suction cups with hooks were affixed to the window 81 cm apart and 30 cm above the water’s edge (the pool adjacent to the window). The left-most suction cup was 163 cm from the left edge of the window. A stimulus board made of 0.64 cm thick plywood and painted white (138 cm high ×122 cm wide) was positioned against the window. The experimenter could look through a narrow opening in the board (5.7 cm high ×59.4 cm wide) to view the otter’s choice. The stimulus board served three purposes: it shielded the experimenter’s body from view as the otter approached the stimuli to prevent inadvertent cueing, it shielded the stimuli from the otter during the inter-trial interval, and it provided a uniform white background that blocked external stimuli from within the building from the otter’s view. The stimuli were suspended on the suction cup hooks and placed behind the board (out of view of the otter) until it was time to begin the trial. During the session, the experimenter stood directly behind the stimulus board and the recorder stood approximately 193 cm behind and to the left side of the experimenter and behind a railing, so as to be out of the view of the otter. Both the experimenter and the trainer carried portable two-way radios (Midland GXT 1000 series, Midland Radio Corporation).
The experimental set-up for Heather. The experimenter stood inside of the building and hung the stimuli from suction cups on the glass window while the otter was on shore with the trainer outside the building. The otter was cued to enter the water and select one of the stimuli by touching the glass window near the stimulus with her nose. The experimenter looked through the slot in the stimulus board and informed the trainer whether the choice was correct or wrong over two-way radios. The trainer positively reinforced the otter with food for correct choices. The recorder stood out of view of the otter in the building and recorded the otter’s choices after each trial. The otter returned to the shore near the trainer during the inter-trial interval
A schematic representation of the experimental set-up we used for Sailor is shown in Fig. 3. The materials were positioned in the experimental area (1 m ×1.5 m). Two 6-cm metal carabiners were affixed to the mesh wall of the enclosure 76 cm apart and 40 cm from the top of the cement wall. The stimuli were suspended from the carabiners using metal paper clips on the outside wall of the enclosure. No stimulus board was used, as the experimenter did not need to stand directly behind the stimuli during trials. The experimenter and recorder stood about 3.5 m to the left of the edge of the experimental area, out of view of the otter to prevent inadvertent cueing. No two-way radios were used, as the trainer was within hearing range of the experimenter and recorder.
The experimental set-up for Sailor. The otter started each trial in the inter-trial interval (ITI) position in the pen adjacent to the experimental area. The otter was cued to enter the experimental area through a gate and select one of the stimuli by rearing onto his hind paws and approaching the stimulus with his nose. The experimenter and recorded stood to the side out of the direct view of the otter. The recorder verbally informed the trainer whether the otter was correct or wrong. The trainer positively reinforced the otter with food for correct choices in the ITI position. The experimenter placed the stimuli in the correct locations clipped to carabiners on the mesh wall of the enclosure
Procedure
Pre-training phase
Heather received pre-training 6 years before the training phase of the current study in which she learned to go to her S+ (with no S- present) when presented with the verbal cue “target.” First, she was trained to approach and touch the 3D red circle held in the trainer’s hand while on exhibit. Then, the 3D red circle was attached to the end of a target pole and moved further away from the trainer until it was held near the glass window of the ECO Center. In the final step, the 3D red circle was presented from inside the ECO Center (a person held the handle on the backside of the stimulus) and Heather was required to swim across the outdoor pool to touch her nose to exterior side of the glass panel directly adjacent to the red 3D circle. This behavior was used repeatedly in summer demonstration programs before and during the training and test phase of the current study.
Sailor did not have the same target training as Heather, but instead his pre-training consisted of viewing his S+ (blue triangle) and his S- (red circle) within his enclosure in the same experimental set-up we used in the training and test-phase. Before beginning a session with Sailor, all enrichment items were removed from the experimental area where the stimuli were positioned and the pen was sprayed with water to clean it. Sailor was gated out of the experimental area and waited in the adjacent pen while two research assistants (an experimenter and recorder) set up the materials. At the start of the first trial for Sailor, the recorder verbally indicated readiness to begin (“ready”). The trainer opened the gate between Sailor’s holding area and the experimental area. Only Sailor had access to these areas during sessions (Heather was not present). Sailor started in the ITI (inter-trial interval) position (see Fig. 3). The trainer cued the otter to approach the stimuli and make a choice by pointing at the stimuli and saying “target.” Sailor then entered the experimental area and indicated his choice by rearing up on his hind paws and positioning his nose near the stimulus he selected (occasionally he touched his forepaw on the enclosure wall or the stimulus as well). Note that Sailor had to view the stimuli in air (unlike Heather, who could view the stimuli in air or underwater). The recorder viewed the otter’s choice and verbally reported to the trainer whether the otter was correct or wrong. If the otter made the correct choice, the trainer would bridge the otter with a clicker, and then offer the otter a piece of fish in the ITI position. If the otter made the wrong choice, the trainer would recall the otter back to the ITI position and offer no fish reward (and no clicker). Immediately after the otter made a choice and retreated to the ITI position, the experimenter placed the stimuli in the correct locations for the next trial. The ITI was approximately 10–20 s. The recorder noted the stimulus selected on each trial, behavioral comments, session start and end time, date, weather, and potential visual or auditory distractions.
During pre-training sessions, the trainer watched Sailor and determined when to place the S+ on the right or left according to his performance in the session to shape his behavior. Initial pre-training sessions included only the S+ (no S- present), or placed the S+ and S- in fixed positions throughout the session according to the trainer’s goals (a modified pseudorandom series (Gellerman, 1933) was not used to determine the location of the S+ during pre-training). Pre-training sessions occurred one to three days per week at 8:30 am (before zoo visitors arrived). Sailor completed 44 pre-training sessions (M = 79%) from June 2016 to November 2016 and April/May 2017. Pre-training continued until Sailor achieved 65% or better in five consecutive sessions.
Training phase
The training phase for this study consisted of selecting the S+ in the presence of the S- in a two-alternative forced choice task in which the position of the S+ on the left or right was determined using a modified pseudorandom Gellerman series (Gellerman, 1933). The S+ was never shown more than two trials in a row on the same side to prevent potential side biases. Each otter viewed only one S+ and S- throughout the training phase (see stimuli section).
At the start of a session for Heather, all otters were removed from the outdoor exhibit area by the zookeeper while the two research assistants (an experimenter and recorder) set up the materials. At the start of the first trial of the session for Heather, the experimenter radioed the trainer to indicate readiness to begin. The trainer then opened the gate to the outdoor exhibit area and the otter and the trainer entered it. Heather was tested individually, and the only otter within the exhibit area during a session. The trainer cued the otter to her start position on a tree stump on the pool edge with a direct path to the stimuli (see Fig. 2). The cue consisted of the verbal prompt “up” with a point to the tree stump. Then the trainer would cue the otter to approach the stimuli and make a choice by pointing at the stimulus board behind the window and saying “target.” The trainer faced away from the stimuli during this time so as not to inadvertently cue the otter to approach the left or right stimulus. The otter dove into the water and swam to the stimuli. The otter indicated her choice by touching her nose to the glass in front of the stimulus she selected. The stimuli were positioned such that they were approximately half-way submerged in the water, so that the otter was able to view the stimuli with her head in or out of the water. The experimenter viewed the otter’s choice and radioed the trainer to report if the otter was correct or wrong. If the otter made the correct choice, the trainer would bridge the otter with a clicker, and throw the otter a piece of fish in the pool. The trainer would then cue the otter to return to shore with the verbal prompt “land” and a pointing gesture. The otter typically ate the fish while swimming back to shore. If the otter made the wrong choice, the trainer would recall the otter back to her station on shore. Immediately after the otter made a choice, the experimenter removed the stimuli from sight by placing them behind the board. Occasionally the trainer would engage the otter in other learned behaviors in between trials to reengage the otter’s attention or motivate the otter after several wrong choices (e.g., wave, stand, target to trainer’s hand). The inter-trial interval (ITI) was approximately 30–60 s. During the ITI, the experimenter placed the stimuli for the next trial in the correct locations, then radioed the trainer to send the otter to the stimulus board again. The recorder noted the otter’s response (stimulus choice), as well as any comments on the otter’s behavior during the trial (trial times were not recorded). For each session the start and end time, the date, the weather, and any potential distractions (e.g., noise from trucks, leaf blowers) were recorded.
Training phase sessions with Sailor were identical to his pre-training sessions with one exception. When the trainer cued the otter to approach the stimuli and make a choice by pointing at the stimuli and saying “target,” the trainer faced away from the stimuli during this time so as not to inadvertently cue the otter to approach the left or right stimulus (in the same manner as was done with Heather). The recorder viewed the otter’s choice and verbally reported to the trainer whether the otter was correct or wrong. This verbal report allowed the trainer to reinforce the otter for correct choices.
Training sessions for both otters occurred 1–3 days per week between 8:30 am and 9:30 am EST (before zoo visitors arrived). This was done to minimize the distractions to the otters, particularly Heather, who had sessions in the public exhibit area (but the public did not view sessions). The otters were trained in dry and light rain conditions (although not during high winds or heavy rains), as long as they were willing to participate in the sessions. No sessions occurred between December and March, as snow and winter conditions made it difficult to conduct sessions. Early training sessions contained 3D stimuli and later training sessions contained 2D stimuli. Each training session typically contained 10–25 trials, depending on the attention and motivation level of the otter and time constraints (M = 15 trials/session for Heather, M = 18 trials/session for Sailor).
Training sessions for Heather occurred from June 2016 to November 2016 (36 sessions with 3D stimuli, four sessions with 2D stimuli), with refresher training sessions in April 2017 prior to the test phase (five sessions with 2D stimuli). Training sessions for Sailor occurred from May 2017 through September 2017 (31 sessions with 3D stimuli, 17 sessions with 2D stimuli). Heather completed 657 training trials and Sailor completed 1,092 training trials. Otters had to achieve 75% correct or better across the last five consecutive training sessions with 3D stimuli before moving on to 2D stimuli. Training continued with 2D stimuli until the otters achieved 75% correct or better on five consecutive sessions.
Test phase
The procedure for the test phase was the same as in the training phase for both Heather and Sailor. Test sessions occurred 1-3 days per week between 8:30 am and 9:30 am EST (before zoo visitors arrived), similar to the training phase. As in the training phase, the otters were tested in dry and light rain conditions (although not during high winds or heavy rains), as long as they were willing to participate in the sessions. Test sessions contained the training stimuli as well as novel test probes (see Table 1). Each test session contained 14 trials (eight trials with training stimuli and six trials with test probes). The first two trials of every test session contained training trials (one trial with the S+ on the right, and one trial with the S+ on the left). The final 12 trials of each session contained six test probes and six training stimuli. All six test probe pairs appeared in each test session. The order of the trials was randomized, with the constraint that the training S+ or test probe stimulus matching the color or shape of the S+ was never shown more than two trials in a row on the same side to prevent potential side biases. The order of the trials was based on a modified pseudorandom Gellerman series (Gellerman, 1933). The training S+ or test probe stimulus matching the color or shape of the S+ appeared equally often on the left and right. On trials with training stimuli, the otters were only reinforced if they chose their S+. On test probe trials, the otters were reinforced for choosing either stimulus. This was done so that the otters would report their preference without learning via feedback.
Test sessions for Heather occurred from May 2017 to November 2017 (48 sessions). There was a break in Heather’s test sessions from 16 July 2017 to 14 September 2017 because she was unwilling to participate in full sessions and developed a pervasive side bias. During this period, we conducted 12 sessions (5–14 trials per session) with only the training stimuli to try to correct the side bias. Heather’s accuracy averaged 85% correct during these 12 interim sessions. Test sessions for Sailor occurred from September 2017 to November 2017 (12 sessions). Sailor did fewer test sessions because he completed pre-training prior to the training phase due to his lack of target training (see pre-training phase above). The test phase ended in November due to winter conditions that prevented further sessions from occurring since both otters were tested outside.
Data analysis
Statistical analyses were performed using R v3.5.0 (R Core Team, 2018) using an experimental type I error level of α = .05. Multilevel mixed-effects logistic regression models were fit using lme4 v1.1-15 (Bates, Maechler, Bolker, & Walker, 2015) due to the longitudinal structure of the experimental design. All p-values were adjusted using Holm’s (1979) method to control the experimental error level at α = .05 except when testing pairwise comparisons for which Tukey’s (1949) HSD was used.
We modeled Heather and Sailor’s data separately because we used different experimental set-ups to test them, and Sailor received fewer test sessions than Heather. Heather and Sailor’s accuracy was modeled with two multilevel mixed-effects logistic regression models. We modeled experimental sessions with random intercepts and tested for fixed effects of the location of the S+ (left side or right side), trial type (training, color probe, shape probe), shape probe stimuli (black circle/triangle, blue circle/triangle, red circle/triangle), and color probe stimuli (red/blue circles, red/blue hexagons, red/blue triangles). We planned on modeling experimenters and recorders with random intercepts, but their estimated variance components were 0 in both models and the multilevel models did not converge; this indicated that there was little variability due to experimenters or recorders, so a simpler pair of models that did not include random effects for experimenter or recorder were used.
Results
Training phase
Overall accuracy during the training phase across all training sessions for both otters (82.9%, SE = 3.9%) was statistically significantly better than chance, Z = 8.4, p < .0001. Sailor’s training performance was 87.5% (SE = 4.8%) and Heather’s training performance was 78.1% (SE = 6.2%). We tested for side biases during the training phase. All p-values were adjusted using Holm’s (1979) method. Both otters exhibited a side bias, although both otters’ performance was better than chance on each side. Heather was significantly more accurate when the S+ was on the right (84%) than when the S+ was on the left (71.7%), Difference = 12.3%, SE = 3.2%, Z = 3.80, p = .0003. Heather’s performance was significantly better than chance when the S+ was on the left (Z = 6.7, p < .0001) and on the right (Z = 10.5, p < .0001). Sailor was significantly more accurate when the S+ was on the left (93%) than when the S+ was on the right (85.3%), Difference = 7.4%, SE = 1.9%, Z = 3.88, p = .0003. Sailor’s performance was significantly better than chance when the S+ was on the left (Z = 18.7, p < .0001) and on the right (Z = 22.6, p < .0001).
Test phase
Heather
After controlling for variability due to sessions, location of the S+, trial type, shape probe stimuli, and color probe stimuli, Heather’s discriminative performance was significantly better than chance (i.e., 50%), Accuracy = 82.6 % , SE = 2.7 % , Z = 9.04, p < .0001, 95 % CI : (77.3%, 87.8%)). The multilevel model was a significantly better fit than an intercept-only model that included only the random session intercepts based on a likelihood ratio test, χ2(7) = 96.3, p < .0001. The intercepts-only model had a modest intraclass correlation, ICC = .13 (Wu, Crespi, & Wong, 2012), which suggested that variability in Heather’s accuracy was explained in part by clustering of the trials in a given session (i.e., there was significant variability in accuracy from session to session).
Likelihood ratio tests were performed to test the statistical significance of fixed effects of S+ location, trial type, shape probe stimuli, and color probe stimuli and the variance component of the random session intercepts; a 50/50 mixture of chi-square distributions with 0 and 1 degrees of freedom was used to test the variance component (Ke & Wang, 2015). All p-values for this set of tests were adjusted using Holm’s method (1979). The variance component for the session intercepts was significantly greater than 0, Deviance = 20.26, p < 0.0001. After controlling for all other effects, Heather was statistically significantly more accurate when the S+ was on the right (Accuracy = 93.7%, 95% CI: (90.0%, 97.3%)) than when the S+ was on the left (Accuracy = 71.5%, 95% CI: (63.2%, 79.7%)), χ2(1) = 53.88, p < .0001. Heather’s performance was significantly better than chance when the S+ was on both the left and the right. There were no statistically significant main effects of shape probe stimuli, χ2(2) = 3.32, p = .3808, and color probe stimuli, χ2(2) = 1.56, p = .4591. This indicates that there were no significant differences in performance among the three shape probe stimuli or among the three color probe stimuli (Table 2).
Controlling for all other effects, there was a significant main effect of trial type, χ2(2) = 26.02, p < .0001. Pairwise comparisons of the three trial types suggested that Heather was significantly more accurate on training stimuli trials (Accuracy = 94.0 % , 95 % CI : (90.3%, 97.6%)) than on color probe trials (Accuracy = 70.6 % , 95 % CI : (55.7%, 85.4%)), Z = 4.84, p < .0001. Heather was also significantly more accurate on training stimuli trials than on shape probe trials (Accuracy = 83.2 % , 95 % CI : (71.6%, 94.8%)), Z = 2.72, p = .0180. There was no significant difference in accuracy between color probe trials and shape probe trials, Z = 1.52, p = .2795. Finally, Heather’s performance was significantly better than chance on all three trial types as all confidence intervals exclude an accuracy of 50%. These results are shown in Fig. 4.
Heather and Sailor’s accuracy shown as a function of trial type: training stimuli, color probes (red/blue circles, red/blue hexagons, red/blue triangles), and shape probes (black circle/triangle, blue circle/triangle, red circle/triangle). Accuracies are shown along with 95% confidence intervals. Chance performance of 50% is shown as a dashed horizontal line
Sailor
After controlling for variability due to sessions, location of the S+, trial type, shape probe stimuli, and color probe stimuli, Sailor’s discriminative performance was significantly better than chance (i.e., 50%), Accuracy = 70.8 % , SE = 5.0 % , Z = 4.05, p < .0001, 95 % CI : (61.1%, 80.6%)). The multilevel model was a significantly better fit than an intercept-only model that included only the random session intercepts based on a likelihood ratio test, χ2(7) = 12.5, p = .4215. The intercepts-only model had a small intraclass correlation, ICC = .03 (Wu, Crespi, & Wong, 2012), which suggested that a small proportion of the variability in Sailor’s accuracy was explained by clustering of the trials in a given session (i.e., there was some variability in accuracy from session to session).
Likelihood ratio tests were performed to test the statistical significance of fixed effects of S+ location, trial type, shape probe stimuli, and color probe stimuli and the variance component of the random session intercepts; a 50/50 mixture of chi-square distributions with 0 and 1 degrees of freedom was used to test the variance component (Ke & Wang, 2015). All p-values for this set of tests were adjusted using Holm’s method (1979). The variance component for the session intercepts was not significantly greater than 0, Deviance = 0.45, p = .7515. After controlling for all other effects, Sailor was statistically significantly more accurate when the S+ was on the left (Accuracy = 80.6%, 95% CI: (70.0%, 91.1%)) than when the S+ was on the right (Accuracy = 61.1%, 95% CI: (47.7%, 74.5%)) , χ2(1) = 7.22, p = .0432. Sailor’s performance was significantly better than chance when the S+ was on the left, but not when the S+ was on the right. Controlling for all other effects, there were no statistically significant differences among different trial types, χ2(2) = 3.73, p = .6200, shape probe stimuli, χ2(2) = 0.01, p = .9942, and color probe stimuli, χ2(2) = 2.00, p = .7515. Like Heather, Sailor showed no significant differences in performance among the three shape probe stimuli or among the three color probe stimuli. Sailor’s performance was significantly better than chance on one of the six stimulus types (see Table 2).
In contrast with Heather’s results, Sailor showed no significant difference in performance among training stimuli trials, color probes, and shape probes. However, pairwise comparisons of the three trial types suggested that Sailor was significantly better than chance on training stimuli trials (Accuracy = 82.7 % , 95 % CI : (71.8%, 93.7%)), but not on color probe trials (Accuracy = 61.6 % , 95 % CI : (32.3%, 90.9%)), or shape probe trials (Accuracy = 68.2 % , 95 % CI : (39.9%, 96.7%)). These results are shown in Fig. 4.
Discussion
The goal of the current study was to explore the ability of North American river otters to learn to discriminate between stimuli using multiple visual features, then test them with stimuli in which one of the features was eliminated. We investigated whether river otters would encode all features present in the training stimuli, and show a difference in performance based on which feature was available to recognize novel test stimuli. Both river otters successfully learned to discriminate between stimuli varying in shape and color. Heather was able to successfully discriminate between novel test stimuli when either color or shape were eliminated as salient features, suggesting that she could use either feature. She showed no difference in performance between shape and color probes, which indicates she had no preference for using either feature. Sailor did not successfully discriminate between novel test stimuli when either color or shape were eliminated as salient features (except for the blue and red hexagons), although he did continue to perform well with training stimuli during the test. Sailor may not have been able to discriminate between the stimuli when we reduced the available features. However, Sailor’s side bias and limited number of test trials likely contributed to his poor performance on the test probes.
Performance differences between Heather and Sailor could have been due to individual differences (e.g., age, sex), Heather’s extended experience with the 3D stimuli, or number of test sessions. No sex differences in performance were found in some cognitive studies with otters (Perdue et al., 2013), while other studies reported sex differences (Frick et al., 2016; Slack, 1966). Age differences were reported in Gormley’s (2015) tool use study with three species of otters, showing that younger animals were more likely to engage with a novel apparatus. In the years prior to the current study, Heather had more experience with the 3D stimuli than Sailor, as she had been target trained, engaged in match-to-sample training (approximately 400 trials), and she was repeatedly exposed to the “target” cue in the presence of her S+ during summer programs at the zoo. However, Sailor did undergo 622 trials of pre-training to develop the “target” behavior. Heather’s experience with the stimuli over many more years may have led her to develop a more robust representation of all the visual features of the stimuli, leading to her better performance during the test. Finally, Heather completed 48 test sessions whereas Sailor completed only 12 test sessions. Sailor started pre-training later than Heather, and entered the training phase later, so there was less time available for test sessions before winter weather ended the study. Sailor may have shown better performance on the test probes if he had the same number of sessions as Heather. However, both Sailor and Heather showed some variability in performance from session to session, which may have been due to their level of alertness prior to a session, their motivation during the session, seasonal behavioral changes, and the weather (they tended to move slower in hotter weather).
Methodological differences could also be a reason for performance differences between the river otters. Due to constraints on interacting with Sailor, we had to use two experimental set-ups to test the otters. We kept the method as similar as possible for the otters, but there were some differences that could have affected the results. First, Heather could view the stimuli in air or underwater, while Sailor was restricted to viewing stimuli in air. This may not have been an issue if L. canadensis possesses equivalent visual acuity in air and underwater like the Asian small-clawed otter (A. cineria; Balliet & Schusterman, 1971). However, the viewing medium may have affected color perception even if it did not impact visual acuity. Second, the Heather could have made her choice at a range of distances from the stimuli at any time from when she jumped in the pool several meters away to when she closely approached the stimuli. Sometimes she swam straight towards her chosen stimulus, and other times she approached the incorrect stimulus and then swerved as the last moment to touch the correct stimulus. Sailor appeared to make his choice while about half a meter or less from the stimuli, sometimes rearing up to peer closely at them. Differences in the experimental set-up made it challenging to compare the otter’s performance, but we did find that both arrangements were effective for testing visual discrimination in otters. In future studies, one could use the set-up we used for Heather to explicitly test underwater visual object recognition (if stimuli are positioned such that they can only be seen underwater). Otters could be trained to position themselves in a hoop station at a fixed position from the stimuli to control viewing distance (as in the sea lion study by Mauck & Dehnhardt, 1997). Alternately, Sailor’s set-up or a similar arrangement (Svoke et al., 2014) is ideal for testing visual discrimination in air.
The current results agree with Slack’s (1966) study showing the ability of two North American river otters to discriminate between shapes, including a circle and triangle. In addition, another mustelid species, the ferret (M. putorius), discriminated between shapes that included an inverted triangle versus an upright triangle and an upright triangle versus a cross (Pollard et al., 1967). Neither Slack (1966) nor Pollard et al. (1967) reported the surface area of their stimuli, and an examination of Fig. 3 of Slack’s (1966) study suggests that the surface area of the stimuli within each pair were not equivalent. The stimuli in the current study were also not equivalent in surface area because we were utilizing stimuli produced by the trainer for target training prior to the current study. The circle had a larger surface area than the triangle. Thus, it is possible that the river otters in the current study as well as the river otters in Slack’s (1966) study were using size as well as shape to discriminate between the stimuli. Given this consideration, we have only preliminary evidence that North American river otters can discriminate shapes and further research is needed. Future experiments should use stimuli that are equivalent in surface area, as well as include a larger variety of shapes.
This study provides preliminary evidence for color perception in North American river otters, but it is not conclusive. A comprehensive test for color perception in a behavioral paradigm requires one to control for brightness cues (Jacobs, 1981). One way to do this is to present each hue in a variety of saturations (measured by percent reflectance as compared to white), and present random pairings of the stimuli (e.g., blue vs. red) such that sometimes one hue is brighter than the other and vice versa. This has been done in “gray-card” experiments where differing shades of gray are tested against different hues as well as the hues tested against each other. Svoke et al. (2014) used this method to show that Asian small-clawed otters (A. cinerea) could discriminate gray from white, green, blue, and red, as well as blue versus green and blue versus red. The current study shows that L. canadensis can possibly discriminate blue versus red as well, but the otters may have been using brightness as well as hue. We did not systematically vary the brightness levels of the stimuli. In addition, the appearance of color can be dependent on the field size, color of the surroundings, luminance level of the color, and luminance level of the surroundings (Padgham & Saunders, 1975). We could not control the luminance level of the surroundings, as we tested outdoors and not in a carefully controlled lab setting. We always tested during daylight hours in the early morning, although we ran sessions in a variety of weather conditions (but not during heavy rain or storms) to ensure we could run enough sessions. There wasn’t an appropriate place to test the otters in carefully controlled conditions in our study. To provide more conclusive evidence for color vision in L. canadensis, future studies should control for brightness and luminance levels, as well as testing more hue comparisons. Furthermore, anatomical tests looking for the presence of functioning cone types should also be done to show L. canadensis possesses color vision.
Both river otters in the current study showed evidence of side biases during both the training and the testing phase. Heather performed better when the S+ was on the right then when it was on the left, although her performance was better than chance on when the S+ was on either side. We observed that Heather often jumped in the pool and swam straight towards the right stimulus, choosing it immediately if it was correct or swerving to the left when she got closer if it was incorrect. This tendency may have caused Heather to inspect the right-side stimulus for longer, or default to choosing right. However, this behavior pattern did not cause her to drop below chance when the S+ was on left. Sailor performed better when the S+ was on the left than when it was on the right in both the training and test phases, and only performed above chance when it was on the left during the test phase. This bias may have been due to the position of the stimuli relative to the doorway where he entered the experimental pen. The right stimulus was closest to the door, and he sometimes appeared to default to choosing the right stimulus quickly instead of viewing both stimuli before making a choice. Side biases have been noted in other cognitive perceptual experiments with otters using a two-alternative forced choice task (Frick et al., 2016; Slack, 1966) as well as in other non-human animals (DeLong, Barbato, O’Leary, & Wilcox, 2017; DeLong et al., 2018; Schleussel, Fricke, & Bleckmann, 2012; Schluessel, Kraniotakes, & Bleckmann, 2014). Side biases may occur when a subject cannot discriminate between stimuli and default to the preferred side in the absence of a successful strategy, or because of other factors. Laterality may account for some side preferences, although we don’t know enough about laterality in otters.
There were only two river otters involved in the current study that completed the test sessions. An additional adult female river otter successfully completed the training phase and learned to discriminate between a yellow square and a red circle, but she did not complete the test sessions due to illness. We utilized all available river otters residing at the Seneca Park Zoo. We should use caution in interpreting results from few subjects because within-species differences between otters can occur (Gormley, 2015; Slack, 1966; Svoke et al., 2014). However, most other perceptual studies have utilized only one to three otters (Balliet & Schusterman, 1971; Gentry & Peterson, 1967; Ghoul & Reichmuth, 2014; Schusterman & Barrett, 1973; Slack, 1966; Svoke et al., 2014). Only rarely are larger groups of otters tested. Perdue et al. (2013) tested eight Asian small-clawed otters, Frick et al. (2016) tested six Asian small-clawed otters, and Schmelz et al. (2017) tested five giant otters and four Asian small-clawed otters. Behavioral studies with otters are typically limited by the number of subjects available at zoos and aquariums. The investment of time and resources to train the otters further limits available subjects based on zookeeper availability and zoo priorities.
Since little research has been done on visual perception in North American river otters, it is unclear to what extent vision plays a role in their daily life. Some research suggests conspecific recognition in L. canadensis likely involves multiple sensory modalities, including vision (Kruuk, 2006). European river otters forage predominantly using vision, supplementing with tactile perception under poor visual conditions (Green, 1977). A recent study suggests that Asian small-clawed otters (A. cinereus) discriminated between social partners using sound and smell, but not vision (Lemasson, Mikus, Blois-Heulin, & Lode, 2013). In the current study, Heather’s superior performance to Sailor may indicate that L. canadensis requires extensive training to succeed on a visual discrimination task. It is possible that L. canadensis is capable of learning to discriminate on the basis of color and shape features, but one or both of those features are not ecologically salient aspects of stimuli.
Studying visual perception in otters is important because very few studies have been done with only a subset of the 13 extant otter species. It is important to gain more knowledge about otter perception since they face threats to their survival including habitat destruction, pollution, hunting and trapping, and vehicle traffic (Yoxon & Yoxon, 2014). According to the International Union for the Conservation of Nature and Natural Resources (IUCN, 2017), seven species of otters are listed as endangered or vulnerable. North American river otters nearly disappeared from widespread regions of many states in the mid-20th century, but several reintroduction projects have succeeded (Kruuk, 2006). In New York, the Seneca Park Zoo, the New York State Department of Environmental Conservation, and local conservation organizations and businesses worked together to reintroduce river otters in the western and central regions of the state. However, river otters are still vulnerable to the same threats they once faced since they inhabit fragile ecosystems (Kruuk, 2006). Global warming is a threat to river otters in that their food supplies are diminishing, and even a small decline in the food supply will have disastrous results (Kruuk, 2006). Knowing more about visual perception in otters could assist conservationists in developing ways to prevent otter death. Perceptual solutions, like the prism system used in Scotland to visually deter otters from crossing a road when a car is approaching, can be used to improve survivability in otters (Yoxon & Yoxon, 2014).
Conducting research with otters residing in zoos and aquariums has benefits for the subjects themselves. Engaging the otters in research sessions serves as an enrichment activity. Carnivores like otters can be susceptible to abnormal behavior patterns when they are under human care because they expend too little effort to obtain food (Carlstead, 1998). Studies have shown enrichment devices or techniques are effective for deterring abnormal behavior patterns in otters (Nelson, 2009; Ross, 2002). Knowing more about visual perception in otters may help zoos design better enrichments devices that capture the otters’ attention. Participating in experimental sessions where complex behavior including a series of actions and decisions is required to obtain food could also be effective in reducing stereotypies in otters. In addition, explaining experiments to zoo and aquarium visitors helps educate people about scientific research and may impact conservation efforts (e.g., Harley et al., 2010). Although our experimental sessions took place out of view of zoo visitors to reduce distractions to the otters, our study goals and findings were communicated to the public during summer programs.
Conclusion
This study was the first to compare and contrast the ability of otters to use different visual features to recognize objects after learning to discriminate between 2D stimuli with multiple features available (color, brightness, shape, size). Both river otters successfully learned to discriminate between stimuli with multiple features available, and one otter was able to successfully discriminate between novel test stimuli when either color or shape were eliminated as salient features. This study provided the first preliminary behavioral evidence for color vision in North American river otters. There have been very few perceptual or cognitive studies done with any species of otter, and only one other study has tested visual object recognition in L. canadensis (Slack, 1966). River otters may use visual object recognition to detect predators, prey, and conspecifics. More research is needed to elucidate salient visual features used by otters.
References
Balliet, R.F. (1970). Color discrimination in the Southeast river otter (Amblonyx cineria cineria) (Unpublished master’s thesis). San Francisco, CA: San Francisco State College.
Balliet, R. F., & Schusterman, R. J. (1971). Underwater and aerial visual acuity in the Asian "Clawless" Otter (Amblonyx cineria cineria). Nature, 234(5327), 305-306.
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1-48. doi:10.18637/jss.v067.i01
Bogale, B.A., & Sugita, S. (2013). Shape discrimination and concept formation in the jungle crow (Corvus macrorhynchos). Animal Cognition, 17, 105-111. doi: https://doi.org/10.1007/s10071-013-0642-y
Carlstead, K. (1998). Stereotypic behaviors in zoo carnivores. In: D.J. Sheperdson, J.D. Mellen, & M. Hutchins (Eds.), Second nature: Environmental enrichment for captive animals.Washington, D.C.: Smithsonian Institution Press.
Cogan, D., Inmam, S., & Gambrel, M. (1971). Discrimination in parakeets (Melopsittacus undulatus) as a function of age. Psychological Record, 21, 117-120
Crimmins, S. M., Roberts, N. M., & Hamilton, D. A. (2009). Effects of prey size on scat analysis to determine river otter Lontra canadensis diet. Wildlife Biology, 15(4), 449-453. doi:https://doi.org/10.2981/08-067
Crowley, S., Johnson, C. J., & Hodder, D. P. (2013). Spatio-temporal variation in river otter (Lontra canadensis) diet and latrine site activity. Ecoscience, 20(1), 28-39. doi:https://doi.org/10.2980/20-1-3509
Daniel, J.C., Mikulka, P.J. (1998). Discrimination learning in the white rhinoceros. Applied Animal Behavior Science, 58, 197-202.
DeLong, C.M. & Barbato, S., O’Leary, T., & Wilcox, K.T. (2017). Small and large number discrimination in goldfish (Carassius auratus) with extensive training. Behavioural Processes, 141(2), 172-183. doi: https://doi.org/10.1016/j.beproc.2016.11.011
DeLong, C.M., Keller, A.M., Wilcox, K.T., Fobe, I., & Keenan, S.A. (2018). Visual discrimination of geometric and complex 2D shapes in goldfish (Carassius auratus). Animal Behavior and Cognition, 5(3), 300-319.
Doty, B. A., Jones, C. N., & Doty, L. A. (1967). Learning-set formation by mink, ferrets, skunks, and cats. Science, 155, 1579–1580.
Fobes, J.L., & Smock, C.C. (1981). Sensory capacities of marine mammals. Psychological Bulletin, 89(2), 288-307.
Fretueg, G. R., Martin, T. J., Widga, C., & Ruez Jr., D. R. (2015). Summer diet characteristics of North American river otters (Lontra canadensis) in central Illinois. The American Midland Naturalist, 173, 294-304.
Frick, E. E., Friedman, L., Peranteau, J., Beacham, K., & Kuczaj II, S. A. (2016). Flexibility and use of a novel tool in Asian small-clawed otters (Aonyx cinerea). International Journal of Comparative Psychology, 29, 1-11. Retrieved from http://escholarship.org/uc/item/74f3c26v
Fuss, T., Bleckmann, H., & Schluessel, V. (2014). Visual discrimination abilities in the gray bamboo shark (Chiloscyllium griseum). Zoology 17(2), 104-111.
Fuss, T., & Schluessel, V. (2015). Something worth remembering: Visual discrimination in sharks. Animal Cognition, 18, 463-471. doi: https://doi.org/10.1007/s10071-014-0815-3
Gallant, D., Vasseur, L., Dumond, M., Tremblay, E., & Bérubé, C. H. (2009). Habitat selection by river otters (Lontra canadensis) under contrasting land-use regimes. Canadian Journal of Zoology, 87(5), 422-432. doi:https://doi.org/10.1139/Z09-035
Gellerman, L.W. (1933). Chance orders of alternating stimuli in visual discrimination experiments. Journal of Genetic Psychology, 42, 206-208.
Gentry, R.L,, & Peterson, R.S. (1967). Underwater vision of the sea otter. Nature, 216, 435-436.
Ghoul, A., & Reichmuth, C. (2014). Hearing in the sea otter (Enhydra lutris): Auditory profiles for an amphibious marine carnivore. Journal of Comparative Physiology A, 200, 967-981. doi: https://doi.org/10.1007/s00359-014-0943-x
Gorman, T. A., Erb, J. D., McMillan, B. R., & Martin, D. J. (2006). Space use and sociality of river otters (Lontra canadensis) in Minnesota. Journal of Mammalogy, 87(4), 740-747.
Gormley, R. (2015). Problem solving and tool use in three species of otter (Unpublished master’s thesis). The University of Tennessee at Chattanooga, Chattanooga, Tennessee.
Green, J. (1977). Sensory perception in hunting otters, Lutra lutra L. Otters: Journal of the Otter Trust, 13–16.
Griebel, U., & Peichle, L. (2003). Colour vision in aquatic mammals - facts and open questions. Aquatic Mammals, 29 (1), 18-30.
Guertin, D. A., Harestad, A. S., & Elliott, J. E. (2009). Summer feeding habits of river otters inhabiting a contaminated coastal marine environment. Northwest Science, 84(1), 1-8. doi:https://doi.org/10.3955/046.084.0101
Hansen, H., McDonald, D.B., Groves, P., Maier, J.A., & Ben-David, M. (2009). Social networks and the formation and maintenance of river otter groups. Ethology, 115(4), 384-396.
Harley, H.E., Fellner, W., & Stamper, M.A. (2010). Cognitive research with dolphins (Tursiops truncatus) at Disney’s The Seas: A program for enrichment, science, education, and conservation. International Journal of Comparative Psychology, 23, 331-343.
Harley, H.E., Roitblat, H.L., & Nachtigall, P.E. (1996). Object representation in the bottlenose dolphin (Tursiops truncatus): Integration of visual and echoic information. Journal of Experimental Psychology: Animal Behavior Processes, 22(2), 164-174.
Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65-70.
IUCN (2017). The IUCN Red List of Threatened Species. Version 2017-3. Retrieved from www.iucnredlist.org
Jacobs, G.H. (1981). Comparative color vision. New York, NY: Academic Press.
Jacobs, G.H. (1993). The distribution and nature of color vision among mammals. Biological Reviews, 68, 413-471.
James, W.T., & McFarland, J. (1966). A study of form discrimination in the opossum. Journal of Psychology, 64, 193-198.
Johnson, M. L. (2000). North American river otter. In P. R. Olofson (Ed.), Baylands Ecosystem Species and Community Profiles: Life Histories and Environmental Requirements of Key Plants, Fish, and Wildlife (pp. 238-240).
Ke, Z., & Wang, L. (2015). Detecting individual differences in change: methods and comparisons. Structural Equation Modeling, 22(3), 382-400. doi:https://doi.org/10.1080/10705511.2014.936096
Kruuk, H. (2006). Otters: Ecology, behavior, and conservation. New York, NY: Oxford University Press.
Larivière, S., & Walton, L. R. (1998). Lontra canadensis. Mammalian Species, 587, 1-8. doi:https://doi.org/10.2307/3504417
Larsen, D. N. (1984). Feeding habits of river otters in coastal southeastern Alaska. The Journal of Wildlife Management, 48(4), 1446-1452. doi:https://doi.org/10.2307/3801818
Lemmason, A. Mikus, M.-A., Blois-Heulin, C., & Lode, T. (2013). Social partner discrimination based on sounds and scents in Asian small-clawed otters (Aonyx cinereus). Naturwissenschaften, 100, 275-279. doi: https://doi.org/10.1007/s00114-013-1022-9
Levenson, D. H., Ponganis, P. J., Crognale, M. A., Deegan, J. F., Dizon, A., & Jacobs, G. H. (2006). Visual pigments of marine carnivores: pinnipeds, polar bear, and sea otter. Journal of Comparative Physiology A, 192(8), 833-843. doi:https://doi.org/10.1007/s00359-006-0121-x
Mauck, B., & Dehnhardt, G. (1997) Mental rotation in a California sea lion (Zalophus californianus). Journal of Experimental Biology, 200, 1309-1316.
Mass, A.M. & Supin, A. Ya. (2000). Ganglion cell density and retinal resolution in the sea otter, Enhydra lutris. Brain Behavior and Evolution, 55, 111-119.
Minini, L., & Jeffery, K.J. (2006). Do rats use shape to solve “shape discriminations?” Learning & Memory, 13, 287-297.
Nelson, K. (2009). Environmental enrichment effects on the activity of a nearctic river otter (Unpublished master’s thesis). Rochester Institute of Technology, Rochester, NY.
Oldfield-Box, H. (1969). On analyzing the formation of learning-sets in young and old rats. Gerontologia, 15, 302-307.
Padgham, C.A. & Saunders, J.E. (1975). The perception of light and colour. New York, NY: Academic Press.
Peichl, L., Behrmann, G., & Kröger, R. H. H. (2001). For whales and seals the ocean is not blue: A visual pigment loss in marine mammals. European Journal of Neuroscience, 13(8), 1520-1528. doi:https://doi.org/10.1046/j.0953-816x.2001.01533.x
Penland, T. F., & Black, J. M. (2009). Seasonal variation in river otter diet in coastal northern California. Northwestern Naturalist, 90(3), 233-237. doi:https://doi.org/10.1898/NWN08-21.1
Perdue, B. M., Snyder, R. J., & Maple, T. L. (2013). Cognitive research in Asian small-clawed otters. International Journal of Comparative Psychology, 26, 105-113. Retrieved from: http://escholarship.org/uc/item/7vv60657
Pollard, J.S., Beale, I.L., Lysons, A.M., & Preston, A.C. (1967). Visual discrimination in the ferret. Perceptual and Motor Skills, 24, 279-282.
R Core Team (2018). R: A language and environment for statistical computing (3.5.0) [programming language]. Vienna, Austria: R Foundation for Statistical Computing, Vienna, Austria. Retrieved from https://www.R-project.org/
Reid, D. G., Code, T. E., Reid, A. C. H., & Herrero, S. M. (1994). Food habits of the river otter in a boreal ecosystem. Canadian Journal of Zoology, 72(7), 1306-1313. doi:https://doi.org/10.1139/z94-174
Ross, S. R. (2002). The effect of a simple feeding enrichment strategy on the behavior of two Asian small-clawed otters (Aonyx cinerea). Aquatic Mammals, 28(2), 113-120.
Schluessel, V., Fricke, G., & Bleckmann, H. (2012). Visual discrimination and object categorization in the cichlid Pseudotropheus sp. Animal Cognition, 15, 525-537. doi: https://doi.org/10.1007/s/1007-012-0480-3
Schluessel, V., Kraniotakes, H., & Bleckmann, H. (2014). Visual discrimination of rotated 3D objects in Malawi cichlids (Pseudotropheus sp.): A first indication for form constancy in fishes. Animal Cognition, 17, 359-371. doi:https://doi.org/10.1007/s10071-013-0667-2
Schmelz, M., Duguid, S., Bohn, M., & Volter, C.J. (2017). Cooperative problem solving in giant otters (Pteronura brasiliensis) and Asian small-clawed otters (Aonyx cinera). Animal Cognition, 20, 1107-1114. doi: https://doi.org/10.1007/s10071-017-1126-2
Schusterman, R.J., & Balliet, R.F. (1970). Conditioned vocalization as a technique for determining visual acuity thresholds in the sea lion. Science, 169, 498-501.
Schusterman, R.J., & Barrett, B. (1973). Amphibious nature of visual acuity in the Asian “clawless” otter. Nature, 244, 518-519.
Schusterman, R.J., Thomas, T. (1966). Shape discrimination and transfer in the California sea lion. Psychonomic Science, 5, 21-22.
Scordino, J. J., Gearin, P. J., Riemer, S. D., & Iwamoto, E. M. (2016). River otter (Lontra canadensis) food habits in a Washington coast watershed: Implications for a threatened species. Northwestern Naturalist, 97(1), 36-47. doi:https://doi.org/10.1898/1051-1733-97.1.36
Serfass, T., M. Rymon, L., & Brooks, R. (1990). Feeding relationships of river otters in northeastern Pennsylvania. Trans. Northeast Sect. Wildl. Soc., 47, 43-53.
Siebeck, U.E., Litherland, L., & Wallis, G.M. (2009). Shape learning and discrimination in reef fish. Journal of Experimental Biology, 212, 2113-2119. doi:10.124/jeb.028936
Sinclair, W., Dunstone, N., & Poole, T.B. (1974). Aerial and underwater visual acuity in the mink Mustela vison Schreber. Animal Behaviour, 22, 965-974.
Slack, R. D. (1966). Learning in the river otter (Lutra canadensis) (Unpublished master’s thesis). Ohio State University, Columbus, OH.
Shlaer, S. (1937). The relation between visual acuity and illumination. The Journal of General Physiology, 21(2), 165-188. doi:https://doi.org/10.1085/jgp.21.2.165
Srinivasan, M.V. (2010). Honeybees as a model for vision, perception, and cognition. Annual Review of Entomology, 55, 267-284. doi: https://doi.org/10.1146/annurev.ento.10908.164537
Sutherland, N.S. (1959). Visual discrimination of shape by octopus. Circles and squares, and circles and triangles. Quarterly Journal of Experimental Psychology, 11, 24-32.
Sutherland, N.S. (1969). Shape discrimination in rat, octopus, and goldfish: A comparative study. Journal of Comparative and Physiological Psychology, 67, 160-176.
Svoke, J. T., Snyder, R. J., & Elgart, J. B. (2014). Preliminary evidence for color stimuli discrimination in the Asian small-clawed otter (Aonyx cinerea). Learning and Behavior, 42,176-184.
Tanaka, K. (2000). Mechanisms of object recognition studied in monkeys. Spatial Vision, 13, 147-163.
Toweill, D. E. (1974). Winter food habits of river otters in western Oregon. The Journal of Wildlife Management, 38(1), 107-111. doi:https://doi.org/10.2307/3800205
Tukey, J. (1949). Comparing individual means in the analysis of variance. Biometrics, 5(2), 99-114.
von Fersen, L., Schall, U., & Gunturkun, O. (2000) Visual lateralization of pattern discrimination in the bottlenose dolphin (Tursiops truncatus). Behavioral Brain Research, 107, 177-181.
Wu, S., Crespi, C. M., Wong, W. K. (2012). Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemporary Clinical Trials, 33, 869-880. doi:https://doi.org/10.1016/j.cct.2012.05.004
Young, M.E., Peissig, J.J., & Wasserman, E.A. (2001). Discrimination of geons by pigeons: The effects of variations in surface descriptions. Animal Learning and Behavior, 29(2), 97-106
Zolman, J.F. (1969). Stimulus preferences and form discrimination in young chicks. Psychological Record 19, 407-416.
Yoxon, P, & Yoxon, G.M. (2014). Otters of the world. Dunbeath, Scotland: Whittles Publishing Ltd.
Author Note
We appreciate that the Seneca Park Zoo allowed us to work with their river otters over an extended period of time. Special thanks to David Hamilton and John Adamski, who provided logistical support and advice throughout the project. David Hamilton provided comments on the manuscript. We thank Kathryn Gardner, David Frye, Jennifer Dami, Trisha Rachfal, Meredith Gamble, and Hannah Thorner for their help running research sessions. In addition, we thank Jennifer Dami for making the schematics showing the two experimental set-ups and Kathryn Gardner for preparing stimuli.
This study was supported by grants from the River Otter Alliance Foundation and the Milwaukee branch of the American Association of Zookeepers. Additional support was provided by the Rochester Institute of Technology College of Liberal Arts and the Rochester Institute of Technology Department of Psychology.
The authors declare no conflict of interest with any of the sponsors of this study. This study was approved by the Rochester Institute of Technology Institutional Animal Care and Use Committee and the Seneca Park Zoo Institutional Animal Care and Use Committee. It was conducted according to the ethical standards of the USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
DeLong, C.M., Wright, C., Fobe, I. et al. North American river otters (Lontra canadensis) discriminate between 2D objects varying in shape and color. Learn Behav 47, 91–104 (2019). https://doi.org/10.3758/s13420-018-0346-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0346-x