Abstract
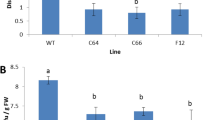
The activity of antioxidant enzymes and ultrastructural changes in tissues inoculated with P. infestans isolate have been studied in the previously developed independent transgenic lines of tomato with FeSOD1 gene and control plants. It is shown that the activity of superoxide dismutase is significantly higher in transgenic plants than that in control plants (nontransgenic plants). Chlorosis and obvious changes in tissue turgor were observed when the control tomato plants were inoculated, which indicates irreversible damages and unimpeded progression of infection. At the same time, the transgenic lines were characterized by the formation of clearly limited zones of damaged cells that rapidly arrested the infection. In addition, the damages differed from those in nontransgenic plants: the cells along the edges of the infection site were smaller and had heavy invaginations of the cell wall. The contacts between the cells were disrupted in this zone, but they were preserved in undisturbed zones of the tissue. Thus, the expression of the FeSOD1 transgene promotes the emergence of the resistance to P. infestans in tomato transgenic plants.
Similar content being viewed by others
References
Thordal-Christensen, H., Zhang, Z., Wei, Y., and Collinge, D.B., Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction, Plant J., 1997, vol. 11, no. 6, pp. 1187–1194.
Zuluaga, A.P., Vega-Arreguin, J.C., Fei, Z., Matas, A.J., Patev, S., Fry, W.E., and Rose, J.K.C., Analysis of the tomato leaf transcriptome during successive hemibiotrophic stages of a compatible interaction with the oomycete pathogen phytophthora infestans, Mol. Plant Pathol., 2016, vol. 17, no. 1, pp. 42–54.
Heat, M.C., Hypersensitive response-related death, Plant. Mol. Biol., 2000, vol. 44, pp. 321–334.
Mur, L.A., Kenton, P., Lloyd, A.J., Ougham, H., and Prats, E., The hypersensitive response: The centenary is upon us but how much do we know? J. Exp. Bot., 2008, vol. 59, pp. 501–520.
Clough, S.J., Fengler, K.A., Yu, I.-C., Lippok, B., Smith, R.K., and Bent, A.F., The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel, Proc. Natl. Acad. Sci. U.S.A., 2000, vol. 97, pp. 9323–9328.
del Pozo, O. and Lam, E., Caspases and programmed cell death in the hypersensitive response of plants to pathogens, Curr. Biol., 1998, vol. 8, pp. 1129–1132.
Montillet, J.L., Chamnongpol., S., Rustérucci, C., Dat, J., Van De Cotte, B., Agnel, J.-P., Battesti, C., Inze, D., Van Breusegem, F., and Triantaphylides, C., Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves, Plant Physiol., 2005, vol. 138, pp. 1516–1526.
Pavet, V., Olmos, E., Kiddle, G., Mowla, S., Kumar, S., Antoniw, J., Alvarez, M.E., and Foyer, C.H., Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis, Plant Physiol., 2005, vol. 139, pp. 1291–1303.
Fobert, P.R. and Després, C., Redox control of systemic acquired resistance, Curr. Opin. Plant Biol., 2005, vol. 8, pp. 378–382.
Torres, M.A., Jones, J.D.G., and Dangl, J.L., Reactive oxygen species signaling in response to pathogens, Plant Physiol., 2006, vol. 141, pp. 373–378.
Liu, Y., Ren, D., Pike, S., Pallardy, S., Gassmann, W., and Zhang, S., Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade, Plant J., 2007, vol. 51, pp. 941–954.
Levine, A., Tenhaken, R., Dixon, R., and Lamb, C., H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response, Cell, 1994, vol. 79, pp. 583–593.
Alvarez, M.E., Pennell, R.I., Meijer, P.-J., Ishikawa, A., Dixon, R.A., and Lamb, C., Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity, Cell, 1998, vol. 92, pp. 773–784.
Sticher, L., Mauch-Mani, B., and Métraux, J.P., Systemic acquired resistance, Annu. Rev. Phytopathol., 1997, vol. 35, pp. 235–270.
Conrath, U., Thulke, O., Katz, V., Schwindling, S., and Kohler, A., Priming as a mechanism in induced systemic resistance of plants, Eur. J. Plant Pathol., 2001, vol. 107, pp. 113–119.
Kamoun, S., Nonhost resistance to Phytophthora: Novel prospects for a classical problem, Curr. Opin. Plant Biol., 2001, vol. 4, pp. 295–300.
Baranenko, V.V., Superoxide dismutase in the plant cells, Tsitologiya, 2006, vol. 48, no. 6, pp. 465–474.
Serenko, E.K., Baranova, E.N., Balakhnina, T.I., Kurenina, L.V., Gulevich, A.A., Kosobruhov, A.A., Maysurian, A.N., and Polyakov, V.Y., Structural organization of chloroplast of tomato plants Solanum lycopersicum transformed by Fe-containing superoxide dismutase, Biochemistry (Moscow) Suppl. Ser. A: Membrane Cell Biol., 2011, vol. 5, pp. 177–184.
Baranova, E.N., Nodel’man, E.K., Kurenina, L.V., Gulevich, A.A., Baranova, G.B., Bogoutdinova, L.R., Akanov, E.N., and Khaliluev, M.R., Ultrastructural organization of chloroplasts and mitochondria of transgenic tomato plants expressing the FeSOD1 gene from Arabidopsis thaliana (L.) Heynh. under salt stress, Russ. Agr. Sci., 2014, vol. 40, pp. 426–431.
Mamonov, A.G., Vasil’chenko, V.V., and Smirnov, A.N., Comparison of P. infestans isolates, collected from potato and tomato plants, for manifestation of aggressiveness on tuber discs of different varieties of potatoes, Izv. S-kh. Akad. im. K. A. Timiryazeva, 2012, no. 5, pp. 61–72.
Khaliluev, M.R., Mamonov, A.G., Smirnov, A.N., Kharchenko, P.N., and Dolgov, S.V., Expression of genes encoding chitin-binding proteins (PR-4) and hevein-like antimicrobial peptides in transgenic tomato plants enhanced resistanse to Phytophthora infestance, Russ. Agric. Sci., 2011, vol. 37, pp. 297–302.
Giannopolitis, C.N. and Ries, S.K., Superoxide dismutases I. Occurrence in higher plants, Plant Physiol., 1977, vol. 59, pp. 309–314.
Nakano, Y. and Asada, K., Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell Physiol., 1981, vol. 22, pp. 867–880.
Reynolds, E.S., The use of lead citrate at high ph as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, pp. 208–212.
Badawi, G.H., Yamauchi, Y., Shimada, E., Sasaki, R., Kawano, N., Tanaka, K., and Tanaka, K., Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts, Plant Sci., 2004, vol. 166, pp. 919–928.
Pavlovskaya, N.E. and Grinblat, A.I., Reactive oxygen species and apoptosis in wheat and peas, S-kh. Biol., 2010, no. 1, pp. 51–55.
Li, J., Luan, Y., and Liu, Z., SpWRKY1 mediates resistance to Phytophthora infestans and tolerance to salt and drought stress by modulating reactive oxygen species homeostasis and expression of defense-related genes in tomato, Plant Cell Tissue Organ Cult., 2015, vol. 123, pp. 67–81.
Tseng, M.J., Liu, C.W., and Yiu, J.C., Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both the superoxide dismutase and catalase in chloroplasts, Plant Physiol. Biochem., 2007, vol. 45, pp. 822–833.
Zurbriggen, M.D., Carillo, N., Tognetti, V.B., Melzer, M., Peisker, M., Hause, B., and Hajirezaei, M.-R., Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria, Plant J., 2009, vol. 60, pp. 962–973.
Higaki, T., Goh, T., Hayashi, T., Kutsuna, N., Kadota, N., Hasezawa, S., Sano, T., and Kuchitsu, K., Elicitor-induced cytoskeletal rearrangement relates to vacuolar dynamics and execution of cell death: in vivo imaging of hypersensitive cell death in tobacco BY-2 cells, Plant Cell Physiol., 2007, vol. 48, pp. 1414–1425.
Soda, N., Singla-Pareek, S.L., and Pareek, A., Abiotic stress response in plants: Role of cytoskeleton, in Abiotic Stress Response in Plants, Tuteja, N. and Gill-Weinheim, S.S., Eds., Wiley-VCH, 2016, pp. 107–129.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.N. Baranova, L.V. Kurenina, A.N. Smirnov, O.O. Beloshapkina, A.A. Gulevich, 2016, published in Rossiiskaya Sel’skokhozyaistvennaya Nauka, 2016, No. 6, pp. 16–21.
About this article
Cite this article
Baranova, E.N., Kurenina, L.V., Smirnov, A.N. et al. Formation of the hypersensitivity response due to the expression of FeSOD1 gene in tomato when it is inoculated with Phytophthora infestans . Russ. Agricult. Sci. 43, 15–21 (2017). https://doi.org/10.3103/S1068367417010049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068367417010049