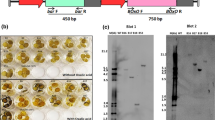
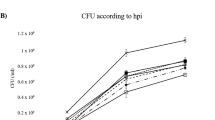
Abstract
H2O2 generated during the oxidative burst, plays important roles in plant defenses responses against pathogens. In this study we examined the role of H2O2 on bacterial canker resistance in transgenic plums over-expressing cytosolic superoxide dismutase. Three transgenic lines (C64, C66 and F12) with elevated levels of H2O2 accumulation showed enhanced resistance against bacterial canker disease caused by Pseudomonas syringae pv. syringae, when compared to the non-transformed control. Analysis of the expression of several genes involved in the plant–pathogen interaction showed that the expression of those involved in SA pathway (pr1 and npr1) and JA (lox3) were activated earlier and transiently in transgenic lines C66 and F12 when compared to the wild type. However, the expression of genes involved in anthocyanin synthesis (chi, chs, f3h, dfr, atcs, myb10) and ethylene (acs) was induced at very low levels whereas it was activated by the pathogen at exaggerated levels in the non-transformed line. These results suggest that resistance observed in transgenic lines over-producing H2O2 is correlated with an early and transient induction of defense genes associated with the SA and JA pathways and inhibition of gene expression associated with ethylene and anthocyanin biosynthesis.








Similar content being viewed by others
References
Kennelly MM, Cazorla FM, de Vicente A, Ramos C, Sundin GW (2007) Pseudomonas syringae diseases of fruit trees: progress toward understanding and control. Plant Dis 91:4–17
Gilbert V, Planchon V, Legros F, Maraite H, Bultreys A (2010) Pathogenicity and aggressiveness in populations of Pseudomonas syringae from Belgian fruit orchards. Eur J Plant Pathol 126:263–277
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Wu G, Shortt BJ, Lawrence EB, Elaine EB, Fitzsimmons KC, Shah DM (1995) Disease resistance conferred by the expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell 7:1357–1368
Wu G, Shortt BJ, Lawrence EB, Leon J, Fitzsimmons KC, Levine EB, Raskin I, Shah DM (1997) Activation of host defense mechanisms by elevated production of H2O2 in transgenic plants. Plant Physiol 115:427–435
Donaldson PA, Anderson T, Lane BG, Davidson AL, Simmonds DH (2001) Soybean plants expressing an active oligomeric oxidase from the wheat gf-2.8 (germin) gene are resistant to the oxalate secreting pathogen Sclerotinia sclerotiorum. Physiol Mol Plant Pathol 59:297–307
Hu X, Bidney DL, Yalpani N, Duvick JP, Crasta O, Folkerts O, Lu G (2003) Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses. Plant Physiol 133:170–181
Dong X, Ji R, Guo X, Foster SJ, Chen H, Dong C, Liu Y, Hu Q, Liu S (2008) Expressing gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus). Planta 228:331–340
Thakur M, Sohal BS (2013) Role of elicitors in inducing resistance against pathogen infection: a review. ISRN Biochem 7:62412
Zhang Z, Collinge DB, Thordal-Christensen H (1995) Germin-like oxalate oxidase, a H2O2-producing enzyme, accumulates in barley attacked by the powdery mildew fungus. Plant J 8:139–145
Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H (1998) An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol Biol 36:101–112
Wang L, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC (2010) An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10:50. https://doi.org/10.1186/1471-2229-10-50
Bari R, Jones JD (2009) Role of plant hormones in plant defense responses. Plant Mol Biol 4:473–488
Robert-Seilaniantz A, Grant M, Jones MD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol 49:317–443
Tsuda K, Katagiri F (2010) Comparing signalling mechanisms engaged in pattern-triggered and effector triggered immunity. Curr Opin Plant Biol 4:459–465
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Faize L, Faize M (2018) Functional use of salicylic acid analogues and their use in crop protection. Agronomy 8:5. https://doi.org/10.3390/agronomy8010005
Faize M, Faize L, Petri C, Barba-Espin G, Diaz-Vivancos P, Koussa T, Rifai LA, Burgos L, Hernández JA (2013) Cu/Zn superoxide dismutase and ascorbate peroxidase enhanced in vitro shoot multiplication in transgenic plum. J Plant Physiol 170:625–632
Block A, Alfano JR (2011) Plant targets for Pseudomonas syringae type III effectors: virulence targets or guarded decoys? Curr Opin Microbiol 14:39–46
Mante S, Scorza R, Cordts JM (1989) Plant regeneration from cotyledons of Prunus persica, Prunus domestica and Prunus cerasus. Plant Cell Tiss Organ Cult 19:1–11
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Bedford KE, Sholberg PL, Kappel F (2003) Use of a detached leaf bioassay for screening sweet cherry cultivars for bacterial canker resistance. Acta Hort 622:365–368
Vicente JG, Roberts SJ (2003) Screening wild cherry micropropagated plantlets for resistance to bacterial canker. In: Iacobellis NS et al (eds) Pseudomonas syringae and related pathogens: biology and genetic. Kluwer Academic Publishers, Dordrecht pp 467–474
Bellicampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122:1379–1385
Tong Z, Qu S, Zhang J, Wang F, Tao J, Gao Z (2012) A modified protocol for RNA extraction from different peach tissues suitable for gene isolation and real-time PCR. Mol Biotechnol 3:229–236
Livak KJ, Schmittgen D (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−Ct method. Methods 25:402–408
Krzesinska EZ, Nina A, Azarenko M (1992) Excised twig assay to evaluate cherry rootstocks for tolerance to Pseudomonas syringae pv. syringae. HortSci 27:153–155
Hammerschlag FA (2000) Resistance responses of peach somaclone 122–1 to Xanthomonas campestris pv. pruni and to Pseudomonas syringae pv. syringae. HortSci 35:141–143
Mgbechi-Ezeri J, Johnson KB, Oraguzie NC (2017) Effect of inoculum concentration, isolates and leaf age on bacteria canker disease development in sweet cherry (Prunus avium L.) cultivars. Acta Hortic 1161:463–468
Mgbechi-Ezeri J, Porter L, Johnson KB, Oraguzie N (2017) Assessment of sweet cherry (Prunus avium L.) genotypes for response to bacterial canker disease. Euphytica 213:145–157
Künstler A, Hafez YM, Király L (2007) Transient suppression of a catalase and an alternative oxidase gene during virus-induced local lesion formation (hypersensitive response) is independent of the extent of leaf necrotization. Acta Phytopathol Entomol Hung 42:185–196
Yi SY, Yu SH, Choi D (1999) Molecular cloning of a catalase cDNA from Nicotiana glutinosa L. and its repression by tobacco mosaic virus infection. Mol Cell 9:320–325
Blackman L, Hardham AR (2008) Regulation of catalase activity and gene expression during Phytophthora nicotianae development and infection of tobacco. Mol Plant Pathol 4:495–510
Hamdoun S, Zhe L, Manroop G, Nan Y, Hua L (2013) Dynamics of defense responses and cell fate change during Arabidopsis–Pseudomonas syringae interactions. PLoS ONE. https://doi.org/10.1371/journal.pone.0083219
Craft KPC, Voisey CR, Slusarenko AJ (1990) Mechanisms of hypersensitive cell collapse: correlation of increased lipoxygenase activity with membrane damage in leaves of Phaseolus vulgaris (L.) cv. Red Mexican inoculated with avirulent races cells of Pseudomonas syringae pv. phaseolicola. Physiol Mol Plant Pathol 36:49–62
Koch E, Meier BM, Eiben HG, Slusarenko A (1992) A lipoxygenase from leaves of tomato (Lycopersicon esculentum Mill.) is induced in response to plant pathogenic and Pseudomonads. Plant Physiol 99:571–576
Adam LA, Juhasz C, Tabias I, Gullner G (2010) Up-regulated expression of lipoxygenase and divinyl ether synthase genes in pepper leaves inoculated with Tobamoviruses. Physiol Mol Plant Pathol 74:387–393
Balaji V, Mayrose M, Sherf O, Jacob-Hirsch J, Eichenlaub R, Iraki N, Manulis-Sasson S, Rechavi G, Barash I, Sessa G (2008) Tomato transcriptional changes in response to Clavibacter michiganensis subsp. michiganensis reveal a role for ethylene in disease development. Plant Physiol 146:1797–1809
Sherif S, Paliyath G, Jayasankar S (2012) Molecular characterization of peach PR genes and their induction kinetics in response to bacterial infection and signaling molecules. Plant Cell Rep 4:697–711
Misyura M, Guevara D, Subedi S, Hudson D, McNicholas PD, Colasanti J, Rothstein SJ (2014) Nitrogen limitation and high density in rice suggest a role for ethylene under high density stress. BMC Genom 15:681. https://doi.org/10.1186/1471-2164-15-681
Sperdouli I, Moustakas M (2012) Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J Plant Physiol 169:576–587
Garcia-Seco D, Zhang Y, Gutirrez-Mañero FJ, Martin C, Ramos-Solano B (2015) Application of Pseudomonas fluorescent to blackberry under field conditions improves fruit quality by modifying flavonoid metabolism. PLoS ONE. https://doi.org/10.1371/journal.pone.0142639
Gutha LR, Casassa LF, Harbertson JF, Naidu RA (2010) Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol 10:187. https://doi.org/10.1186/1471-2229-10-187
Wang N, Zhang Z, Jiang S, Xu H, Wang Y, Feng S, Chen X (2016) Synergetic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissu Organ Cult 127:217–227
Feng S, Wang Y, Yang S, Xu Y, Chen X (2010) Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 232:245–255
Gharibi S, Tabatabaei BES, Saeidi G, Talebi M, Matkowski A (2019) The effet of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 162:260–269
Shafi A, Kumar Pal A, Sharma V, Kalia S, Kumar S, Singh Ahuja P, Kumar Singh A (2017) Transgenic potato plant overexpressing SOD and APX exhibit enhanced lignification and starch biosynthesis with improved salt stress tolarance. Plant Mol Biol Rep 35:504–518
Shafi A, Gill T, Zahoor I, Sing Ahuja P, Sreenivasulu Y, Kumar S, Kumar Singh A (2019) Ectopic expression of SOD and APX genes related to secondary cell wall cellulose biosynthesis and improve salt tolerance. Mol Biol Rep 46:1985–2002
Acknowledgements
This work was supported by the Spanish Ministry and Competitiveness (Project CICYT BFU2009-07443). MF was supported by the University Chouaib Doukkali.
Author information
Authors and Affiliations
Contributions
MF designed the research, performed the experiments and wrote the manuscript. LF carried out the experiments, prepared tables and figures and participated in data analyses. JSV participated in data analysis and manuscript writing. NA and LB contributed to the transformation experiments and performed statistical analyses.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faize, M., Faize, L., Alburquerque, N. et al. Hydrogen peroxide generated by over-expression of cytosolic superoxide dismutase in transgenic plums enhances bacterial canker resistance and modulates plant defence responses. Mol Biol Rep 47, 5889–5901 (2020). https://doi.org/10.1007/s11033-020-05660-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05660-8