Abstract
Additive manufacturing, popularly known as “3D printing”, enables us to fabricate advanced scaffolds and cell-scaffold constructs for tissue engineering. 4D printing makes dynamic scaffolds for human tissue regeneration, while bioprinting involves living cells for constructing cell-laden structures. However, 3D/4D printing and bioprinting have limitations. This article provides an up-to-date review of 3D/4D printing and bioprinting in tissue engineering. Based on 3D/4D printing, 5D printing is conceptualized and explained. In 5D printing, information as the fifth dimension in addition to 3D space and time is embedded in printed structures and can be subsequently delivered, causing change/changes of the environment of 5D printed objects. Unlike 3D/4D printing that makes passive/inactive products, 5D printing produces active or intelligent products that interact with the environments and cause their positive changes. Finally, the application of 5D printing in tissue engineering is illustrated by our recent work. 3D/4D/5D printing and bioprinting are powerful manufacturing platforms for tissue engineering.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the long human history, two-dimensional (2D) printing has made immeasurable impacts on various aspects of our society, including education, economy, science, and engineering. In the 1980s, printing technologies started to have revolutionary developments, advancing from 2D printing to 3D printing. 3D printing began when Hull patented the world’s first 3D printer, stereolithography (SLA), in 1986 in the USA. Over the past 30 years, many other 3D printing technologies have been developed, including selective laser sintering (SLS), 3D powder binding (3DPB), fused deposition modeling (FDM), extrusion-based 3D printing, and inkjet printing. Nowadays “3D printing”, formally known as “additive manufacturing (AM)”, has been a term representing a group of technologies with which materials (or “inks” in AM) are deposited in a layer-by-layer manner to form 3D objects, non-porous or porous. Compared to conventional subtractive manufacturing, 3D printing has many distinctive advantages, including customized construction of complex 3D objects with high precision, much reduced material waste in product manufacture, and shortened or reduced product development cycles. Owing to these unique advantages, 3D printing has already made great achievements in the biomedical engineering field [1]. Tissue engineering, an essential part of biomedical engineering, has inevitably benefited hugely from the application of 3D printing. 3D printing has often been adopted to fabricate a variety of tissue engineering scaffolds with individualized structures and characteristics by using different types of biomaterials including polymers, ceramics, metals and their composites [2, 3]. For example, Xie et al. used selective laser melting (SLM) to produce porous biodegradable Mg-based implants, which showed excellent antibacterial property and osteoinductivity when using a rabbit model [4]. Furthermore, 3D printing has advanced to allow the construction of complex tissues or organs (e.g., skin, bone, and cartilage) by depositing living cells, biomolecules and biomaterials in a layer-by-layer manner, leading to an new, exciting biofabrication platform, i.e., 3D bioprinting [5]. In the work by Gao et al., a bioink composed of hydrogels, poly(3,4-ethylenedioxythiophene):sulfonated lignin (PEDOT:LS), and neural stem cells was 3D bioprinted into cell-laden spinal scaffolds, which could promote neuronal differentiation of the stem cells in vitro and regeneration of spinal cord in vivo [6]. Although highly useful in tissue engineering, one major limitation of 3D printing and 3D bioprinting is that they only produce static objects in relation to time. 3D printed or bioprinted products are assumed to stay static during their whole application periods, which has limited their usefulness in tissue engineering.
To overcome the limitations of 3D printing and bioprinting, within the past 10 years, printing technologies have further advanced to 4D printing by setting time as the fourth dimension in addition to the traditional three dimensions of space. The concept of 4D printing was introduced in 2013 by Tibbits of the Self-Assembly Lab at Massachusetts Institute of Technology (MIT), USA. At that time, 4D printing was defined as “3D printing plus time”. After the development of nearly a decade, one popular definition of 4D printing currently is that the shape, property and/or functionality of 4D printed structures can change under appropriate stimuli such as temperature, pH, water, electromagnetic radiation, etc., during their application [7]. Since its first introduction, 4D printing has gained increasing attention in many industries and has opened up a new R&D area for tissue engineering. 4D printing offers a new, powerful manufacturing platform and can produce dynamic tissue engineering structures which can stay in their temporary shapes before application and then change to their permanent states under suitable stimuli after deployment, thereby meeting the demanding requirements in some particular clinical situations. A shape memory cycle for 4D printed shape memory polymer structures is illustrated in Fig. S1. Briefly, a 4D printed product of a desired or designed temporary shape is programmed to fix its permanent shape at a temperature above its transition temperature (Tt) of the material, i.e., the melting temperature (Tm) of a semi-crystalline polymer or the glass transition temperature (Tg) of an amorphous polymer. After cooling down to below Tt, the permanent shape can be returned to the desired or designed temporary shape. The temporary shape and permanent shape of a 4D printed structure can be changed reversibly through heating and cooling, leading to a shape memory cycle of the 4D printed structure. To achieve such desirable shape transformations, careful consideration must be given to certain parameters in 4D printing, including effective parameters (e.g., programming and recovery temperatures, heating rate and cooling rate), quantitative parameters, and 4D printing parameters (e.g., shape fixity, shape recovery, and stress recovery) [8, 9]. For example, polyvinyl chloride (PVC), a shape memory polymer with a Tg of about 70 °C, was 4D printed using FDM [9]. The 4D printed PVC was reshaped under three different temperatures [cold (Tg – 45 °C), warm (Tg – 15 °C), and hot (Tg + 15 °C)] and two load holding times (0 s and 600 s) under three-point bending and compression modes. The results showed that changes in reshaping temperature and load holding time affected shape fixity and recovery, as well as stress recovery [9]. However, 4D printing makes products that only have changes for or in themselves but do not affect their environments, which can also limit their usefulness in tissue engineering.
Over the time in our history, printing technologies have advanced from 2D printing to 3D printing and now to 4D printing. Each of these advances was accompanied by the increase in physical dimensions, i.e., from 2D planes to 3D space, or by the extension into time, i.e., from 3D space to 4D’s “3D space plus time”. As information and its influences are essential nowadays in every parts of our lives, information can therefore be set the fifth dimension to advance 4D printing to 5D printing, entering a new phase of additive manufacturing [10]. Unlike 3D printing, which uses 3D space, and 4D printing, which extends from 3D space into time, 5D printing employs not only 3D space and time but also information. On the basis of 4D printing, 5D printing embeds and utilizes information in printed products. Information, the fifth dimension, can be species that will cause changes in the environment upon their release to achieve what 5D printing aims for. 5D printing will enable the fabrication of shape-morphing and information-embedded-and-disseminating objects, which should be highly useful for tissue engineering with the aim of achieving the best clinical outcomes.
In this mini-review and perspective article, recent advances in 3D/4D printing in tissue engineering are reviewed and discussed in terms of printing technologies, materials, and applications. On the basis of 3D/4D printing and their developments, the concept of 5D printing is explained and illustrated, and the potential and prospects of 5D printing in tissue engineering are discussed.
Tissue engineering and strategies
Tissue engineering, as a multidisciplinary field that integrates engineering, life science and clinical medicine, offers an exciting, vital and promising approach that can provide artificial biological substitutes for diseased or injured human body tissues or organs, thereby maintaining, restoring, and/or improving tissue and organ functions. In this section, the concept and development of tissue engineering and strategies for tissue engineering are briefly presented and discussed.
Concept and development
Organ transplantation is used ultimately to save the life of patients with critical tissue loss or organ failure. It has made great successes in clinical medicine by replacing dysfunctional tissues or organs in patients with normal, functional ones from donors. However, organ transplantation faces many challenges, including shortage of donors, possible disease transmission, and rejection of a foreign tissue or organ by patients’ immune system. Against this background, tissue engineering came up strongly over 30 years ago and has since been developed as a vital and promising approach that provides artificial biological substitutes for diseased or lost tissues or organs. The term “tissue engineering” was first proposed in the mid-1980s and was used to describe technologies that could be used in operations for tissues and organs during surgery [11]. In 1987, “tissue engineering” was conceptualized as an multidisciplinary field that applied the principles of engineering and life science to understand relationships of function-structure of human tissues and organs and to develop biological substitutes to restore, maintain or improve tissue functions [12]. There are generally three approaches in tissue engineering: scaffold-based, growth factor-based, or cell-based tissue engineering. In scaffold-based tissue engineering, there are three major components: cells, scaffolds, and biological signals, which are the so-called “tissue engineering triad”. These three components are necessary for mimicking the three basic elements of native human tissues, namely, cells, extracellular matrix (ECM) and bioactive biomolecules/growth factors. ECM provides a structural support for cell attachment, proliferation, differentiation and communication, while biological molecules give specifical signals/guidance for cells and newly formed tissues for their behavior and functions. Cells of the targeted tissue, or stem cells in recent years, are essential for new tissue formation.
Scaffold-based tissue engineering
Among the three approaches in tissue engineering, scaffold-based tissue engineering has been dominantly used for regenerating human body tissues. Tissue engineering scaffolds act as artificial ECM and provide a good substrate for cell attachment and growth and facilitate neo-tissue formation [13, 14]. There are several major requirements for tissue engineering scaffolds in regenerative medicine. The top priority is the biocompatibility of scaffold material and hence the scaffold, i.e., a biocompatible scaffold that can co-exist with the host tissue without any side effect and should elicit a desirable response from the host tissue after implantation. Secondly, scaffold should be highly porous, and the pores should be interconnected, which will allow ingrowth of neo-tissues and hence facilitate tissue regeneration. Thirdly, in the conventional sense for tissue engineering, scaffolds should be biodegradable; and an ideal biodegradable scaffold is expected to have degradation kinetics comparable to the formation rate of the new tissue during its regeneration. Additionally, mechanical properties of scaffolds should be adequate for device handling and implantation. In many situations, they need to be tailored to match those of target tissues to offer an environment with suitable stress for neo-tissue formation. In addition, bioactive biomolecules may be loaded relatively easily into the scaffold matrix or onto the surface of scaffold struts for promoting tissue regeneration in vitro and in vivo [15, 16]. For example, Zhang et al. incorporated two small biomolecules (i.e., resveratrol and strontium ranelate) into 3D printed poly(ε-caprolactone)/hydrogel composite scaffolds [17]. Their results showed that the dual release of these small molecules had combinational advantages in enhancing angiogenesis and inhibiting osteoclast activities. Furthermore, advanced tissue engineering scaffolds should have an architecture that mimics the structure of ECM (pore shape, pore size, porosity, etc.) to influence positively cell behavior (attachment, spreading, etc.) and to enable deep cell penetration/migration [6, 18]. In the work by Zhang et al., 3D printing was applied to construct bone tissue engineering scaffolds with integrated hierarchical Haversian bone structure [19]. Their results showed that the Haversian bone-mimicking scaffolds contributed to the osteogenic, angiogenic, and neurogenic differentiation in vitro and speeded up the blood vessel ingrowth and new bone formation in vivo.
To obtain a scaffold that meets the aforementioned requirements, biomaterials, biomaterial processing, scaffold fabrication, and/or material and scaffold modification are critical factors and should be carefully considered, chosen and studied. Common biomaterials used for producing scaffolds include biomedical polymers, suitable biodegradable metals, bioceramics, and their composites [11, 14]. These biomaterials can be used based on specific tissue engineering applications. For example, metallic biomaterials possess excellent mechanical properties which are suitable for orthopedic applications where long-term load bearing is required [20]. Apart from biomaterials, scaffold fabrication techniques are another important issue related to scaffold-based tissue engineering, and they greatly determine the final characteristics/properties of the scaffold, such as pore size, porosity and mechanical properties. A variety of scaffold fabrication techniques have been developed and utilized to make tissue engineering scaffolds with different types of biomaterials. These fabrication techniques include solvent casting and porogen leaching, melt molding, gas foaming, freeze-drying, fiber bonding, and electrospinning [21, 22]. However, most of these techniques have limitations in fabricating scaffolds with controlled architectures and functionalities for regenerating target tissues. 3D printing and 4D printing, as powerful manufacturing platforms, allow the fabrication of various scaffolds with complex geometries and high precision by accurately placing biomaterials, biomolecules, and/or living cells in the 3D structures. On top of this, post-printing modifications (e.g., coating, surface chemical modification, etc.) for printed scaffolds can also be conducted to further improve the performance of these scaffolds.
Technologies and materials for 3D/4D printing and bioprinting
At present, a range of 3D/4D printing and bioprinting technologies have been used to produce diverse tissue engineering scaffolds with different types of biomaterials. In this section, 3D/4D printing and bioprinting technologies and related materials are presented and discussed.
3D printing technologies
According to the ISO/ASTM standard [23], 3D printing technologies for tissue engineering can be divided into seven categories: (1) binder jetting (e.g., 3D powder binding (3DPB)), (2) direct energy deposition (e.g., laser engineered net shaping (LENS)), (3) material extrusion (e.g., fused deposition modeling (FDM), micro-extrusion printing), (4) material jetting (e.g., inkjet printing, micro-valve jetting, laser-assisted jetting, Polyjet), (5) powder bed fusion (e.g., selective laser sintering (SLS), selective laser melting (SLM), electron beam melting (EBM)), (6) sheet lamination (e.g., laminated object manufacturing (LOM)), and (7) vat photopolymerization (e.g., stereolithography (SLA), digital light processing (DLP)). Each 3D printing technology has its own specific features, advantages and limitations, and can be selected for its use based on the specific tissue engineering application, as summarized in Table 1.
When these 3D printing technologies are applied for tissue engineering, they follow a similar designing and fabrication processes, as illustrated in Fig. 1. Firstly, the medical imaging data of the diseased/injured tissue or organ of a patient are collected via a modern medical imaging technology such as computed tomography (CT) and magnetic resonance imaging (MRI). These medical imaging data are then processed using computer-aided design (CAD) software to transform them into a corresponding virtual model, which is further sliced into a series of 2D layers (with each layer corresponding to a contour of the virtual model) using softwares installed in 3D printers. These softwares are usually proprietary assets of 3D printer makers. Next, the 3D printing machine reads the sliced data and prints out a tissue engineering product using a suitable “ink” or “bioink”—inks are acellular, while bioinks contain living cells. In some situations, 3D printed tissue engineering structures need to undergo post-printing treatment(s) to obtain final products or to improve their performance (removing sacrificial materials to create hollow interconnected channels, sintering to improve mechanical properties, etc.). Finally, after culturing and maturation, 3D printed tissue engineering structures are implanted in patients to repair their dysfunctional tissues or organs.
As for 4D printing technologies, in principle, any 3D printing technology that can process 4D printing inks can be used for 4D printing. 4D printing inks are the inks that involve so-called “smart materials” and 4D printed structures from these materials can change their shapes or properties under suitable stimuli during application [24]. At present, 3D printing technologies commonly used for 4D printing in tissue engineering are primarily micro-extrusion-based printing [25], FDM [26], SLA [27] and DLP [28].
With regard to bioprinting, not all 3D printing technologies for tissue engineering are suitable since living cells are involved during the bioprinting process. For example, 3D printing technologies such as FDM and SLS use high temperatures in their fabricating processes, which will lead to the total destruction of cells, and therefore cannot fabricate 3D functional living structures [26, 29]. At present, 3D printing technologies commonly used for bioprinting are micro extrusion-based printing [21], inkjet printing [30], laser-assisted jetting [31], SLA [32] and DLP [33]. These 3D printing technologies have mild or acceptable processing/fabrication environments, which allow them to incorporate living cells and biological molecules during biomanufacturing.
Materials for 3D/4D printing in tissue engineering
Although 3D/4D printing has made great advances in tissue engineering over the past two decades, developing suitable materials for 3D/4D printing in tissue engineering is still one of the major challenges. Each year, numerous researchers around the world make their efforts to develop biomaterials that have excellent biocompatibility, printability, biodegradability, and suitable mechanical properties for 3D printing in tissue engineering [5]. The paramount property for biomaterials for 3D printing in tissue engineering is biocompatibility. The second most important property of these biomaterials is their printability for the 3D printing technology concerned. For 4D printing, biomaterials should also have stimuli-responsive properties, which will enable 4D printed structures to change their shape or properties when exposed to external stimuli during applications.
For 3D printing in tissue engineering, currently available biomaterials can be classified into four categories: biomedical polymers, metallic biomaterials, bioceramics, and biomedical composites, as presented in Table 2. Among them, biomedical polymers are the most commonly used biomaterials for 3D printing in tissue engineering [11]. According to their sources, polymers can be classified as natural polymers and synthetic polymers. Most of natural biomedical polymers exhibit excellent biocompatibility and biodegradability but have poor mechanical properties. Synthetic biomedical polymers show much improved mechanical properties and slower degradation in comparison with natural polymers but lack bioactive sites that are desirable for tissue engineering applications. Therefore, chemical modification or blending of polymeric biomaterials is often performed to avoid their respective shortcomings. For example, gelatin cannot maintain a stable structure at 37 °C as it is in a liquid-like state at this temperature. Blending gelatin with alginate can form hydrogel blends with improved extrudability and fast crosslinking ability and avoid this issue [34]. Currently, 3D printing can process both natural and synthetic biomedical polymers into diverse tissue engineering products for the regeneration of body tissues such as bone [13, 35, 36], articular cartilage [16, 37, 38], tendon and ligament [39, 40], and vasculature [22, 41,42,43,44]. Metallic biomaterials have excellent mechanical properties and hence are very attractive for repairing tissues and organs where long-term load bearing is required. However, many metallic implants face the problem of too high stiffness than the target tissue (mainly, bone), leading to stress shielding and consequently implant failure. To mitigate the stiffness problem for implantable metal products, 3D printing can fabricate porous metallic tissue engineering scaffolds with different porosities [45]. However, processing metals via 3D printing is often not easy. Currently available 3D printing technologies for metals are SLM, EBM and LENS. These technologies have been used to process different metallic biomaterials into scaffolds for bone [45,46,47] and osteochondral tissue [20]. Bioceramics can elicit specific responses from the host tissue, which makes them very appealing for bone tissue engineering applications. However, 3D printed pure bioceramic products are normally brittle. Currently, bioceramics such as hydroxyapatite (HAp) and β-tricalcium phosphate (β-TCP) are often added into 3D printed bone tissue engineering scaffolds to improve their osteoconductivity [3, 18, 19, 48, 49]. As can be seen, single-phase biomaterials generally have various weaknesses which prevent them from fully meeting the requirements in tissue engineering. To overcome this problem, two or more different types of biomaterials can be combined to produce composites with features that cannot be obtained from existing single-phase biomaterials alone [50]. A composite is made of at least a major phase (i.e., the matrix) and a minor phase (i.e., the so-called reinforcement), which are bonded by an interface between the two phases. Its properties are greatly affected by characteristics of the matrix and reinforcement, distribution and orientation of the reinforcement, and the interfacial state. Even though there are major difficulties, 3D printing of biomedical composites has been increasingly conducted to manufacture different scaffolds or cell-scaffold constructs with improved performance. For example, poly(ε-caprolactone) (PCL) nanofiber was incorporated into hyaluronic acid (HA) hydrogel to generate a composite with improved mechanical properties [51]. The composite scaffolds produced exhibited a microarchitecture mimicking the extracellular matrix of soft tissues, higher bulk porosity and cell permeability, which led to enhanced soft tissue reconstruction.
For 4D printing in tissue engineering, “smart materials” are a key component but currently they are still very limited as 4D printing itself is still at its early development stage. The commonly used smart materials for 4D printing include shape memory polymers, shape-morphing hydrogels, shape memory metal alloys, and composite materials, as summarized in Table 3. A shape memory polymer has a transition temperature (Tt) and once heated above Tt, the polymer structure will change to its permanent shape. For example, our group has used poly(d,l-lactide-co-trimethylene carbonate) (PDLLA-co-TMC) to fabricate shape memory scaffolds for bone or vasculature tissue engineering [52, 53]. For shape-morphing hydrogels, their shape-change ability is due to their reversible large volume change caused by shrinking or swelling when water molecules leave or enter polymer networks in hydrogels. Using 4D printing, such hydrogels can be printed into a series of 2D patterns which have uneven swelling/shrinking ratios among the different parts of the hydrogel membranes, leading to the transformation of these 2D patterns into 3D structures when immersed in water. Heterogenous swelling/shrinking of hydrogel structures can be prepared with different components across the hydrogel thickness [28, 54, 55], with different components across the hydrogel plane [25], or with different components across both the thickness and plane of a hydrogel [56, 57]. For example, our group 4D printed alginate/methylcellulose (Alg/MC) hydrogels into a series of 2D patterns that could transform into different simple and complex 3D structures by responding to water, as shown in Fig. S2 [25]. For shape memory metal alloys, under external thermomechanical stimuli, they can recover to original shapes after severe pseudoelastic deformation. Their shape memory ability is due to the transformation of martensitic phase to austenitic phase in the metals. Currently, most shape memory alloys are based on Ni–Ti compositions. With regard to composites, just like composites for 3D printing in tissue engineering, a good number of composites have been investigated for obtaining improved performance for 4D printing in tissue engineering. For example, incorporation of aligned nanofibers into a hydrogel endowed the material with stiffness and swelling anisotropy and therefore resulted in shape-morphing ability [58]. The addition of Fe3O4 nanoparticles into a shape memory polymer rendered the material responsive to electromagnetic stimuli [59], which is useful for remote actuation applications.
Materials and cells for bioprinting
Since living cells are involved in bioprinting, the selection of suitable biomaterials is a very critical factor for achieving successful bioprinting to create 3D biofunctional living structures. A biomaterial for bioprinting should not only meet the requirements for tissue engineering but also be able to maintain good cell viability during bioprinting and promote cell growth after bioprinting. Currently, biomaterials available for bioprinting are mainly hydrogels and decellularized extracellular matrix (dECM) [60]. Both types of biomaterials have their own advantages and limitations. Most hydrogels are biocompatible, printable, crosslinkable and low-cost and hence are the most used biomaterials in bioprinting. Currently, there are a range of hydrogels for bioprinting, including alginate, collagen, gelatin, GelMA, HAMA, agarose, silk fibroin, PEG and its derivatives, etc. [60]. For example, Duan et al. bioprinted a bioink composed of HAMA, GelMA, and human aortic valvular interstitial cells into trileaflet valve conduits with good shape fidelity [61]. They showed that the cells grew well in the hydrogels during a 7-day in vitro culture. However, the microenvironments provided by these hydrogels for cell growth are not the same as those of native body tissues, which may limit cell growth and functionality. Compared to hydrogels, dECM prepared from body tissues or organs provides a better biomimicking microenvironment for cells after bioprinting. In the work by Choi et al., skeletal muscle-derived dECM-based bioinks were 3D bioprinted into muscle constructs, which were demonstrated to provide a myogenic environment that supported good cell growth, differentiation, and maturation [62]. However, the preparation of dECM is difficult and hence dECM is normally expensive, which limits their applications in bioprinting for tissue engineering. Therefore, every year researchers around the world still make great efforts to develop new biomaterials for bioprinting.
Apart from biomaterials, cells are another critical factor for achieving successful bioprinting. Based on the cell source, cells used for bioprinting can be somatic cells or stem cells that are isolated from patients. Somatic cells are terminally differentiated cells that can be isolated and proliferated in vitro for bioprinting for repairing the target tissue or organ. However, somatic cells are difficult to isolate, possess relatively poor proliferation ability and have short lifetime, which limit their applications in bioprinting for tissue engineering. Furthermore, somatic cells of the target tissue or organ of a patient may be diseased and hence cannot be used for tissue repair. Under such circumstances, allogeneic somatic cells may provide an alternative; but they may cause host immune rejection after implantation. Therefore, stem cells isolated from patients are increasingly chosen and used in bioprinting. Stem cells in the undifferentiated state exhibit good proliferation ability and can differentiate into targeted somatic cells. Stem cells commonly used in bioprinting are human bone marrow- or adipose-derived mesenchymal stem cells (MSCs), amniotic fluid-derived stem cells, and induced pluripotent stem cells (iPSCs). These stem cells can be selected based on the target tissues/organs that need to be regenerated.
In bioprinting, a bioink can be formed by combining a biomaterial and living cells or simply by using living cells themselves. Each strategy has its own pros and cons. A bioink composed of a biomaterial and living cells normally shows good printability for printing out designed 3D structures with high precision. Additionally, the biomaterial can provide mechanical and structural support for cell growth before new ECM formation in vivo. However, cell density, viability, interaction and functionality need careful consideration and subsequent investigations when cells will be encapsulated in a material matrix. Cell viability may show large differences among different hydrogels, such as alginate, agarose and Pluronic® F-127 [63]. In addition, the addition of cells may affect the properties (such as rheological properties and photocrosslinking ability) of the biomaterial in the bioink, requiring carefully consideration when using a cell-laden material as a bioink [64]. For bioprinting of living cells solely in the form of cell aggregates (i.e., cell spheroids and organoids), 3D cellular structures with high cell density, good cell viability and excellent cell–cell interaction can be achieved. These bioprinted cellular structures should have an excellent fusion with the host tissues after implantation. However, the preparation of cell aggregates is time-consuming and not easy. Furthermore, simple cell solutions have poor printability and are thus very difficult to be printed into complex and precise 3D structures. In addition, the initial bioprinted structures solely composed of living cells do not possess sufficient mechanical strength to support cell growth. However, such cellular structures could gain improvements later when new ECM is formed from them in vivo.
Various bioinks have been prepared/developed by different researchers for bioprinting to create tissues and organs with desirable properties and pattern fidelity. For bioprinted structures, the properties (mechanical properties, degradation kinetics, etc.) of bioinks affect their physical properties and also the cells in the bioinks which will provide the structures with biological functions. The pattern fidelity of the bioprinted constructs is closely related to the printability of bioinks, which are different for different bioprinting technologies. For micro extrusion-based bioprinting, bioinks should possess excellent rheological properties (i.e., shear viscosity and viscosity recovery), which allow them to be extruded as good filaments to form 3D structures with precise patterns [65, 66]. For inkjet bioprinting, the viscosity, surface tension and density of a bioink should be carefully controlled to generate droplets with good shape [30]. For SLA and DLP, bioinks should be photocrosslinkable and flowable for building 3D structures [28, 67]. Efforts have been continuously made to improve the printing resolution and pattern precision of bioprinted constructs. Gong et al. enhanced the resolution of extruded hydrogel constructs by immersing them in a solution with opposite net charges after printing, where the electrostatic attraction caused hydrogel shrinkage and led to increased resolution by several factor [68]. Making use of the thermo-reversible gelling properties of Pluronic F127, Zheng et al. used inkjet bioprinting to produce high-resolution microvascular structures with a minimum feature size of 30 μm [69]. You et al. developed bioinks for DLP by combining iodixanol, photocrosslinkable hydrogels and living cells [33]. The inclusion of iodixanol greatly reduced the light scattering during DLP-bioprinting, achieving high cell density (0.1 billion cells/ml) and high-resolution (50 µm) 3D bioprinting of vascularized tissues [33].
Applications of 3D/4D printing and bioprinting in tissue engineering
3D/4D printing has rapidly increased their applications in tissue engineering for fabricating diverse acellular scaffolds and cell-laden structures. In this section, the applications of 3D/4D printing and bioprinting for tissue engineering are presented and discussed.
Skin
Skin is the largest organ of the human body and has a multilayered structure consisting mainly of epidermis, dermis, and hypodermis. In recent studies (Table S1), most researchers fabricated 3D structures mimicking the structure of native skin via 3D printing of different biomaterials. The inks or bioinks for printing skin substitutes are mainly based on hydrogels and their composites, such as collagen, GelMA, and strontium silicate particle reinforced hydrogel blend. Other biomaterials such as dECM, PCL and PVA have also been used in 3D printing of tissue engineered skin. In some of these cases, gelatin was used as a sacrificial material to fabricate hollow channels to serve as vasculature for the printed skin structures [70]. Living cells used for bioprinting of skin products are keratinocytes and fibroblasts, in which keratinocytes are used to form the epidermal layer and fibroblasts are used to form the dermal layer. Endothelial cells may also be used in 3D printing of skin tissue engineering to generate vascularized skin. It has also been reported that the addition of stem cells into the printed skin structures could accelerate the healing of skin wounds [71]. Biomolecules such as epidermal growth factor can be used to promote the differentiation of stem cells and hence improve the repair of skin wounds.
There are several strategies for fabricating skin products via 3D printing. The first common strategy is to directly print out designed skin substitutes containing skin-related cells [71,72,73,74]. For example, Zhou et al. used DLP to process photocrosslinkable hydrogel blends into bilayer structures, with the bottom layer containing endothelial cells and the top layer containing fibroblasts (Fig. 2) [73]. They found that cells migrated and interacted with each other after culture for several days. The printed skin structures also showed good performance in repairing the dermal layer at skin wound sites. The other common strategy is to firstly create a dermal layer structure with or without blood vessels, followed by seeding or inkjet printing of epidermal keratinocytes onto the dermal layer to generate multilayered skin structures [70, 75, 76]. For example, Barros et al. firstly bioprinted a bioink containing GelMA, alginate and endothelial cells to form a vascular network layer, then poured fibroblast-containing GelMA onto the vascular layer to form the dermal layer, and finally seeded multilayered keratinocytes onto the dermal layer to develop a 3D skin substitute with layers of endothelial cell networks, dermal fibroblasts, and multilayered keratinocytes [75]. In recent years, in situ bioprinting has been applied for skin repair by directly depositing cell-laden bioinks onto skin defects or wound sites, which was shown to improve the integration between the bioprinted products and surrounding skin tissue and hence accelerated skin regeneration [77, 78].

Reproduced with permission from Biomaterials, 258, 120287 (2020). Copyright 2020 Elsevier Ltd. [73].
Fabrication of functional living skins (FLS) with interconnected microchannels via DLP for skin regeneration. (a) Schematic diagram and photograph for DLP; (b) illustration for the bilayer scaffold with a top layer containing human skin fibroblast (HSFs) and a bottom layer containing human umbilical vein endothelial cells (HUVECs) and cell migration within the scaffold; (c) cell migration of HUVECs (stained in green) and HSFs (stained in red) printed in FLS and co-culture after 1, 3, and 5 days (the area between two lines indicated the distance of cell migration, scale bar: 200 μm); (d) schematic illustration for the FLS for full-thickness skin defect repair in a rat model; (e) photographs showing skin healing for four rat groups within 21 days (control: non-treatment; hydrogel: injection of GelMA/modified HA solution to the wound site and then UV crosslinking; scaffold: cell-free FLS).
Bone
3D printing has been heavily investigated for bone tissue engineering and has fabricated different bone scaffolds. Currently, there are still numerous new reports on 3D printing of bone tissue engineering scaffolds, as shown in Table S2. A variety of bone tissue engineering scaffolds with improved performance have been produced via 3D printing of different types of biomaterials. Bioactive biomolecules are also added to printed scaffolds to accelerate bone regeneration. The commonly used biomolecules include bone morphogenic protein-2 (BMP-2) [79] and osteogenic peptide (OP) [48]. In addition, other biomolecules such as resveratrol and strontium ranelate have been explored in 3D printing of bone tissue engineering, showing positive effects on promoting bone formation [17]. Apart from bone-related biomolecules, vasculature-related biomolecules such as vascular endothelial growth factor (VEGF) and angiogenic peptide are also added in 3D printed bone scaffolds to enhance angiogenesis, which facilitates nutrient and oxygen transfer and waste clearance during bone regeneration [36]. Currently, cells used in 3D printed bone scaffolds are osteoblastic cells and bone marrow-derived mesenchymal stem cells (BMSCs). The aforementioned biomolecules that are incorporated in bone tissue engineering scaffolds can induce osteogenic differentiation of BMSCs.
In recent years, 4D printing in bone tissue engineering has gathered pace. 4D printing allows us to fabricate shape memory scaffolds that can change their shapes to match the size and shape of bony defects in the body after their deployment. A few shape memory biomaterials such as soybean oil epoxidized acrylate [80] and PDLLA-co-TMC [81] have been used for 4D printing in bone tissue engineering. As shown in Fig. 3, our group has developed cryogenic 4D printing to process PDLLA-co-TMC-based composites into shape-morphing scaffolds for bone tissue engineering [53]. The cells and biomolecules used in 4D printed bone scaffolds are generally the same as those used in 3D printing for bone tissue engineering.

Reproduced with permission from Biofabrication, 12 (4), 045025 (2020). Copyright 2020 IOP Publishing Ltd [53].
In vivo regeneration of bone at rat bone defect site by using 4D printed PDLLA-co-TMC-based scaffolds. (a) Schematic illustration of the shape morphing process of 4D printed scaffolds after deployment under the stimuli of heat generated by near-infrared irradiation; (b) μ-CT images of cranial bone regeneration with different scaffolds; (c) bone volume/total volume (BV/TV) ratio, and (d) bone mineral density of regenerated bone tissue. (Control, TS, BTS, PTS, BPTS refer to non-treatment, TCP/β-TCP/PDLLA-co-TMC, OP/β-TCP/PDLLA-co-TMC, BPN/β-TCP/PDLLA-co-TMC and BPN/OP/β-TCP/PDLLA-co-TMC scaffold, respectively).
Articular cartilage and osteochondral tissue
Repairing articular cartilage and osteochondral tissue is very challenging because the former lacks vasculature and the latter is anisotropic in composition and properties and has a layered structure comprising articular cartilage and subchondral bone. Nevertheless, the application of 3D printing for articular cartilage or osteochondral tissue regeneration has been extensively explored, as shown in Table S3. There are a range of biomaterials for 3D printing in regenerating these two tissues, such as hydrogel blends [38, 82], fiber-reinforced hydrogels [83, 84], particle-reinforced hydrogels [37, 85]), dECM [86, 87], and synthetic polymers [49, 85, 88, 89]. For example, Chae et al. used meniscus-derived dECM that contained human bone marrow MSCs to produce meniscus constructs for cartilage regeneration, as illustrated in Fig. 4 [87]. In addition to biomaterials, TGF-β1 is a commonly used growth factor for improving cartilage regeneration. For repairing defected osteochondral tissue, bone-related biomolecules such as BMP-2 and OP can be used to promote the repair of lesions in subchondral bone. Cells used in 3D printing in this area are chondrocytes and MSCs. Under the stimuli of appropriate biomolecules, MSCs can be induced to differentiate into chondrocytes.

Reproduced with permission from Biomaterials, 267, 120,466 (2020). Copyright 2020 Elsevier Ltd. [87].
3D bioprinted meniscus using meniscus-derived dECM (me-dECM) bioink containing human bone marrow MSCs. (a, b) Schematic overview of the fabrication process; (c) 3D printed meniscus constructs for rabbit, beagle, and human models; (d-f) in vivo chondrogenic efficacy of 3D bioprinted meniscus in the rat model (d: H&E staining; e: safranin O staining; f: type II collagen (COL2) staining; primed me-dECM: 1-week in vitro chondrogenic culture before implantation).
There are major differences between fabricating acellular scaffolds or living structures via 3D printing for articular cartilage and osteochondral tissue. Hydrogel-based and dECM-based inks or bioinks are often made for 3D printing for articular cartilage. In addition, fibrous inks or bioinks involving nanofibers are popular in recent years to mimic the collagen fiber component in native articular cartilage [51, 83, 86, 88, 90, 91]. As for 3D printing for osteochondral tissue, heterogeneous scaffolds with composition, structure and mechanical strength gradients have been designed and fabricated because of the multi-layered structure of osteochondral tissue that exhibits different mechanical properties for each layer. Normally, synthetic polymers with good mechanical properties are used for fabricating the subchondral bone part, while the cartilage part is made from hydrogel-based or dECM-based inks or bioinks.
Tendon and ligament
Tendon and ligament are fibrous tissues in human bodies. Tendon connects muscle to bone, while ligament connects bone to bone. In terms of composition and structure, they are not very different from each other. So far, a few 3D printing technologies have been used to process different polymers and their composites into tissue engineering scaffolds for tendon and ligament regeneration, as shown in Table S4. Common polymers for 3D printing for tendon and ligament applications are PCL [40, 92, 93], PLGA [39] and PLA [94]. Pitaru et al. used several commercial filaments to print out 3D constructs and compared their mechanical properties with native anterior cruciate ligament (ACL), showing none of these biomaterials were optimal for ligament regeneration due to unmatched mechanical properties [94]. Jiang et al. designed and then fabricated via 3D printing two types of PLGA scaffolds with collagen-fibrin hydrogels containing MSCs, showing the feasibility of both types of scaffolds for tendon regeneration (Fig. 5) [39]. Biomolecules such as TGF-β3 have been used in tendon scaffolds to induce MSCs differentiation. Cells used for 3D printing for tendon and ligament regeneration include tendon stem/progenitor cells, tenocytes, fibroblasts, and MSCs.

Reproduced with permission from Bioactive Materials, 5, 634–643 (2020). Licensed under a Creative Commons Attribution (CC BY) license. [39].
3D printing of two types of tendon scaffolds with PLGA and collagen-fibrin hydrogels. (a, c) Schematics of these two types of scaffolds, and (b, d) corresponding photographs; (e, f) H&E staining of the two types of scaffolds after two-week implantation using a mouse model, (e) and (f) corresponded to scaffolds (a) and (c), respectively (* representing the solid end of the scaffold, scale bar: 500 μm).
Muscle
Producing muscle substitutes that mimic the structural and functional characteristics of native muscles in the body is an appealing strategy for repairing damaged muscles. Table S5 shows recent studies on 3D/4D printing for muscle tissue engineering. Currently, biomaterials for 3D printing for muscle regeneration are dominantly hydrogels and their composites, such as nanoclay/GelMA [95] and HA/gelatin/fibrinogen [96]. For example, Kang et al. used micro extrusion-based 3D printing to process a bioink composed of phenol-rich gelatin (GHPA), GO and C2C12 skeletal myoblasts into muscle tissue engineering scaffolds, showing good cell proliferation and myogenic differentiation of C2C12 skeletal myoblasts (Fig. 6) [97]. In another study, dECM derived from muscle was investigated for 3D printed muscle structures [62]. It was also shown that incorporation of VEGF into 3D printed scaffolds could improve the repair of volumetric muscle loss [95]. Cells for 3D/4D printing for muscle substitutes are mainly myoblasts. It was found that the addition of neural stem cells into muscle-related cells could assist muscle cell proliferation, leading to improved muscle regeneration [96]. Furthermore, 4D printing has been used in muscle tissue engineering to fabricate shape-morphing structures. An example is the work by Ionov and co-workers, in which melt electrowriting and extrusion-based printing were combined to fabricate self-scrolled bilayer structures which could support the proliferation and alignment of muscle cells for muscle tissue engineering [98, 99].

Reproduced with permission from ACS Macro Letters, 10, 426–432 (2021). Copyright 2021 American Chemical Society [97].
3D bioprinting using a bioink composed of phenol-rich gelatin (GHPA), GO and C2C12 myoblasts for muscle regeneration. (a) Photograph of sol–gel transition of Go/GHPA hydrogels; (b) schematic of the 3D printing process; (c) photograph of the printed structure; (d) illustration of the in-situ crosslinking procedure for GO/GHPA hydrogels; (e) myogenic differentiation of C2C12 within the GO/GHPA hydrogels [myosin heavy chain stained with FITC (green); F-actin stained with TRITC (red); nucleus stained with DAPI (blue)].
Blood vessel
Vasculature is very important for the body to maintain cell viability and functionality in the lifetime by providing nutrient transfer, oxygen exchange, waste removal, and signaling factors regulation. Table S6 summarizes recent applications of 3D/4D printing in vasculature tissue engineering. For 3D printing of blood vessels, there are several strategies for fabricating vascular channels. The first strategy is to use co-axial nozzles to print out tubular hollow channels [41, 42, 68, 100, 101]. In a co-axial 3D printing head, two ejection channels, one inner and one outer, are available in the co-axial nozzle. The inner channel is un-fed or is fed with a sacrificial material (e.g., gelatin) or a crosslinking solution (e.g., CaCl2 solution) and the outer channel is fed with a biomaterial (crosslinked or to be crosslinked) that can support the tubular structure. The second strategy is to use a sacrificial core to print out a 3D structure with interconnected hollow channels [22, 65, 102, 103]. In some studies, the sacrificial material was firstly 3D printed, followed by casting of the matrix material, and finally removal of the sacrificial material to obtain a structure that contained interconnected channels [22, 68]. In other studies, the sacrificial core and support part were both 3D printed by alternate printing of a sacrificial ink and a support material [65, 102]. Furthermore, synchronous 3D printing of sacrificial and support bioinks has been performed to fabricate structures with nutrient microchannels, as shown in Fig. 7 [104]. After printing, the sacrificial core would be removed to obtain a 3D structure with interconnected channels. The third strategy is to use SLA or DLP to directly fabricate structures with interconnected channels according to CAD designs [43]. The cells used for 3D printing of vasculature are mainly endothelial cells. Biomolecules such as VEGF and heparin can be incorporated in 3D printed scaffolds to promote blood vessel regeneration.

Reproduced with permission from Advanced Healthcare Materials, 9, 1,901,142 (2020). Copyright 2020 John Wiley and Sons [104].
Fabrication of structures containing interconnected microchannels via synchronous 3D bioprinting of a sacrificial core (gelatin) and crosslinkable GelMA. (a) Schematic illustration of the reversable thermo-crosslinking of gelatin and unreversible crosslinking of GelMA; (b) illustration of the 3D printing process; (c, d) photographs of the 3D printed structures and confocal laser scanning microscopy (CLSM) images of cross-sections, showing the microchannels; (e, f) CLSM images of MC3T3 and HUVECs within in the bioprinted structures.
4D printing has also been applied, which fabricates shape-morphing vascular scaffolds. These scaffolds can change their shape from 2D planar shape to 3D tubular shape by responding to suitable stimuli. Currently, popular biomaterials for 4D printing of vasculature are shape-morphing hydrogels and shape memory polymers. For example, alginate MA [54], HAMA [54], GelMA [55, 56] and silk [28] have been 4D printed into water-responsive self-folding tubes with an inner diameter ranging from 20 μm to several millimetres for vascular tissue engineering. Our work provides an example of using shape memory polymer PDLLA-co-TMC to produce shape-morphing vascular scaffolds [52]. The 4D printed PDLLA-co-TMC scaffolds could automatically fold into 3D tubular structures when heated to the human physiological temperature. Zhang et al.’s work provides another example, in which thermo-responsive poly(glycerol dodecanoate) acrylate (PGDA) was 4D printed into heat-responsive self-tubing polymeric scaffolds for regenerating vasculature, as shown in Fig. S3 [105].
Neural tissue
The neural tissue, including the central nervous system (CNS) and peripheral nervous system (PNS), plays a key role in the regulation of physiological functions and human body activities. Because of its high complexity, neural tissue repair is highly difficult, particularly for CNS. At present, neural tissue engineering scaffolds are mainly made for PNS repair and can be fabricated using techniques such as phase separation [106] and electrospinning [107]. As shown in Table S7, 3D/4D printing has also been widely applied to process different synthetic polymers and hydrogels into customized neural tissue engineering scaffolds via a few 3D printing techniques, such as micro-extrusion printing and DLP. Other techniques such as near-field electrostatic printing, melt electrowriting, and electrohydrodynamic 3D printing appeared to be usable for fabricating scaffolds. As shown in Fig. 8, Liu et al. used micro-extrusion based bioprinting to process neuron stem cell-laden hydrogels into neural tissue constructs to treat spinal cord injury [108]. Some conducting materials such as graphene [109] and graphene oxide [110] could be added into scaffolds to improve their conductivity and hence to accelerate neural tissue regeneration. Biomolecules such as SDF-1 and retinoic acid (RA) can be loaded in scaffolds to induce the proliferation and differentiation of stem cells. Commonly used cells for neural tissue engineering are PC-12 cells, neural stem cells and iPSCs. In recent years, 4D printing has also been explored for neural tissue engineering. For example, Miao et al. used SLA to process soybean oil epoxidized acrylate and graphene into multi-responsive scaffolds for neural tissue engineering [109]. Their scaffolds could reshape into tightly scrolled tubular structures and provide good support for the growth and differentiation of MSCs, exhibiting good potential for PNS repair.

Copyright 2021 Elsevier Ltd [108].
3D bioprinted neural tissue for spinal cord regeneration. (a) Schematic illustration for 3D bioprinting of spinal cord-like scaffolds; (b) photographs of (i) printed structure and (ii) in vivo implantation of scaffold into the gap of the lesion area of a rat with spinal cord injury (SCI); (c) survival and differentiation of neural stem cells (NSCs) after three-month implantation (i: GFP (green) and Tuj1 (red) to show the survival and neuronal differentiation; ii: GFP (green) and NF (red) to show the survival and mature neuron formation; iii: GFP (green) and Oligo2 (red) to show the survival and oligodendroglia differentiation). (HBC: hydroxypropyl chitosan; HA-SH: thiolated hyaluronic acid; HA-VS: inyl sulfonated hyaluronic acid; MA: Matrigel.) Reproduced with permission from Biomaterials, 272, 120,771 (2021).
Bladder
The bladder is a hollow muscular distensible organ that stores urine from kidneys before its disposal by urination. People having bladder diseases or cancers can suffer significantly with a low quality of life. It was reported in 2018 that all over the world, there were 549,393 new patients suffering from bladder cancer while males accounted for 424,082 of the new cases [111]. However, bladder repair or regeneration is difficult due to its complex, multi-layered and highly elastic structure. Research and reports on 3D printing of bladder scaffolds are still rather limited, which may be due to the challenge of finding or developing biomaterials with excellent biocompatibility, biodegradability, printability and highly elastic properties, together with the difficulties in fabricating heterogeneous multi-layered constructs similar to the structure of native bladder. Hydrogels such as collagen have been used to fabricate cell-laden bladder scaffolds [112]; but most of the hydrogels exhibited very poor mechanical properties which are far below those of the native bladder. Wang et al. used coaxial electrospinning and 3D printing to fabricate bilayer scaffolds composed of a heparin-loaded electrospun layer and a 3D printed PCL layer [113]. The application of these bilayer scaffolds enhanced bladder regeneration in a rat model, as illustrated by Fig. S4.
Liver
The liver is the largest gland in the human body and regulates a variety of functions such as metabolism, bile production, and detoxification. Owing to its complex micro-architecture and multiple types of cells, the total recreation of the native liver microenvironment is highly challenging, if possible. 3D printing provides a powerful and promising platform for fabricating liver substitutes. In vitro liver microtissues or models have been made from diverse hydrogels or liver dECM using different 3D printing technologies (e.g., DLP, micro-extrusion) [114, 115]. Several studies have focused on fabricating multiscale liver lobules with multiple types of cells and interconnected vasculatures, aiming to obtain biomimetic cell-laden constructs similar to the highly complex native liver [116, 117]. For example, Janani et al. used a dual-nozzle extrusion-based bioprinting system to process two dECM-based bioinks into liver models that mimicked microarchitecture of native liver lobule [117], which had high potential for drug toxicity and screening applications. Also, liver organoids could be engineered into 3D liver constructs for disease modelling and transplantation [118]. For example, Yang et al. used a micro-extrusion based bioprinter to fabricate hepatorganoids, which were transplanted into mice with liver failure and improved the survival of these mice, as illustrated by Fig. S5 [119]. Despite the advances of 3D printing in liver tissue engineering, fabrication via 3D printing or other means of liver substitutes comparable to the native liver with extensive vasculature, lobes, lobules, hepatocytes, and sinusoids is still a daunting task and remains as one of the highest hurdles in regenerative medicine.
Heart
3D bioprinting of a functional, full-size whole heart as that in humans must be among the few greatest challenges for engineering and medicine owing to the highly vascularized, complex structures and multiple functions of the heart, particularly the eternal beating of the heart of a living person. In recent years, 3D bioprinting has been used to fabricate some relatively simple constructs for heart parts (e.g., heart valve), patches or “mini-organs”. Materials used for 3D printing of the heart are mainly hydrogels (e.g., collagen [120], alginate [34], GelMA [61]) and dECM [121]). There are various types of cells that can be used for 3D printing of heart-related constructs such as leaflet interstitial cells, smooth muscle cells, endothelial cells, cardiomyocytes, MSCs and iPSCs. In 2015, a new 3D printing technique, i.e., freeform reversible embedding of suspended hydrogels (FRESH), was developed and applied to process low-viscosity hydrogels into full-size models of the human heart [120, 122]. As shown in Fig. S6, an organ-scale human heart could be made via FRESH 3D bioprinting from low-viscosity collagen [120]. In addition, 4D printing has been used to fabricate dynamic heart constructs. For example, Wang et al. used DLP to process a 4D ink composed of a shape memory polymer and graphene into near-infrared light-sensitive cardiac constructs with highly aligned microstructure and adjustable curvature [123]. This 4D printed cardiac construct was expected to recreate the curved topology of the myocardial tissue for its seamless integration with the heart. Despite the progress in 3D printing of heart constructs, there are still many difficulties for producing heart tissue substitutes that are comparable to the natural heart, including the creation of the whole set of blood vessels of the heart, large number of cells required to rebuild a human-size heart, and long-term in vitro culture.
Other tissues and organs
In addition to the tissues and organs that have been presented and discussed above, 3D/4D printing has also been used to create scaffolds or constructs for other human body tissues/organs, such as cornea [124], gastrointestinal tract [125, 126] and kidney [127]. For example, Kong et al. used electric field direct writing to fabricate microfibrous scaffolds and then infused the scaffold with GelMA hydrogels to obtain fiber-hydrogel composites, which mimicked the stromal structure of native cornea and provided a good environment for the regeneration of corneal stroma [124]. Brassard et al. used organoid bioprinting to fabricate macro-scale tubular intestinal epithelia with in vivo-like crypts and villus domains [125]. Also, kidney organoids were used to manufacture uniformly patterned kidney tissue sheets via micro extrusion-based bioprinting, and these tissue sheets exhibited the potential for renal repair [127].
Challenges for 3D/4D printing in tissue engineering
Although additive manufacturing has made remarkable progress in tissue engineering, it still faces many challenges and some of these challenges may be insurmountable within the foreseeable future. The first challenge is to develop new biomaterials for 3D/4D printing in tissue engineering, particularly for 4D printing. A suitable biomaterial for 3D printing in tissue engineering should have good biocompatibility, printability, biodegradability, and mechanical properties [1, 5, 60]. Additionally, biomaterials for 4D printing should also have good stimulus-responsive properties which will allow 4D printed structures to change shape or properties under appropriate stimuli [24]. Another challenge is to develop new 3D/4D printing technologies with improved performance for tissue engineering applications. Current 3D printing technologies still have various, technology-specific limitations, such as printing speed, printing resolution, and multiple material printing in some printers (e.g., SLA and SLM), which has limited their further applications in producing newer and better tissue engineering products. Next, for 3D/4D bioprinting, there are still many problems for this type of new technologies in creating clinically usable biological substitutes or products. Cell issues such as cell source, cell number/density, cell viability, and cell spreading within the matrix should be carefully considered and investigated to produce clinically good cell-laden constructs for tissue regeneration. Requisite cell density and microstructural complexity must be met for producing and achieving large and functioning tissues or organs [121]. Furthermore, building up the entire blood vessel network for whole organs such as liver and heart and incorporating them into the 3D/4D printed organs are a huge challenge that must be tackled, in the short run rather than in the long run. Finally, 3D or 4D printing itself has limitations, i.e., the static structure issue in 3D printing and the environment effect issue in 4D printing, which can prevent 3D/4D printed structures from meeting the highly demanding requirements for tissue regeneration, remain. Such issues may be mitigated by moving AM forward into 5D printing.
5D printing and its application in tissue engineering
Based on the concepts and developments of 3D printing and 4D printing, Wang in his invited talk at the 2021 Materials Research Society Spring Meeting & Exhibit introduced the concept of 5D printing and presented a research work showing the application of 5D printing in tissue engineering [10]. In this section, the concept of 5D printing is presented in more detail and its application is illustrated by 5D printing in tissue engineering.
Concept and practice of 5D printing
In 3D printing, objects (non-porous or porous) are produced through the layer-by-layer precise deposition of materials in 3D space (i.e., in the traditional, physical three dimensions in space). 3D printed objects are static during their whole product lifetime (i.e., both manufacture and service time). In 4D printing, time is added as the fourth dimension, and then dynamic structures are fabricated by using 3D printing technologies and smart materials, as well as, in many cases, smart designs. 4D printed objects will undergo shape or property change(s) by responding to pre-determined external stimuli. But 3D/4D printing produces passive or inactive products which do not interact with the environment. On the basis of 3D printing and 4D printing, it should be feasible to add another dimension, the fifth dimension (which can be “information”), to 4D printing to move the additive manufacturing platform forward to 5D printing which will fabricate active or intelligent structures that interacts with the envirenment and causes possitive changes. Information nowdays plays a dominent role in our society and it is therefore natural to chose information as the fifth dimension for 5D printing. Here, information is defined in a broad sense and can be any species that will lead to the change/changes of the environment of 5D printed objects (or, the 5D printed objects themselves) upon their release to achieve what 5D printing aims at for individual applications. Therefore, 5D printing can be defined as:
5D printing produces shape-morphing and information-embedded structures, and the information, which is the 5th dimension in 5D printed structures, will be delivered in situ during applications of these structures. More importantly, with 5D printed structures, the in situ delivered information will affect the surrounding environment (or, the 5D printed structures themselves) and guide the change/changes in the environment (or, 5D printed structures). Unlike 3D/4D printing which makes passive or inactive products that can fulfill intended functions but do not change the environment, 5D printing produces active or intelligent products that interact with the environments and cause their intended, positive changes.
In 5D printing, information is involved in addition to 3D space and time for additive manufacturing. As such, 5D printed structures can not only change their own shape or properties during their applications by responding to suitable stimuli but also change the environment (or, the structures themselves) upon the release of embedded information, which will lead to wider applications of printing technologies and printed structures in diverse industries. Unlike 3D/4D printied passive or inactive structures that only provide the spceific functon/functions and hence simply serve the purspose(s), 5D printed active or intelligent structures will fulfill the functions and importantly, cause positive changes of their environments.
5D printing in tissue engineering
As discussed in the "Applications of 3D/4D printing and bioprinting in tissue engineering" section of this article, 3D/4D printing has now been extensively used in tissue engineering for making a wide range of acellular scaffolds and biological substitutes with desired architectures and properties for regenerating different body tissue and organs. With the introduction of 5D printing, tissue engineering products with much improved performance are expected. When applying 5D printing in tissue engineering, the fifth dimension, i.e., information, can be biomolecules such as growth factors (GFs). It can also be other entities (functional nanoparticles, genes, cell messages, etc.). Biomolecules are commonly used in tissue engineering to promote cell proliferation and differentiation and facilitate new tissue formation. Therefore, with the encapsulation of bioactive biomolecules, tissue engineering products can accelerate the regeneration of target tissues. With 5D printing, novel tissue engineering products with shape-morphing ability and controlled delivery of information could be developed for regenerating complex body tissues. In this section, practical application of 5D printing is illustrated through an example in the tissue engineering field, with the aim of developing novel multi-layered tissue engineering products mimicking the native tissue for improving tissue regeneration outcome.
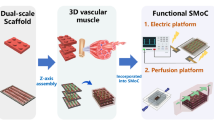
Scaffold-based tissue engineering provides an important route for fabricating biological substitutes for tissue maintenance, restoration and improvement. 3D printing has enabled efficient and reproducible fabrication of 3D, biocompatible, biodegradable and complex scaffolds, which can serve as an artificial ECM environment and temporary support for new tissue formation and growth. Furthermore, 4D printing allows development of dynamic tissue engineering products which can change their shapes or properties under suitable stimuli to fit the anatomical geometry or functions of the target tissues after their deployment in the body. For 5D printing, the illustrative example here is the investigation and development of novel multi-layered cell-laden constructs for generating tubular tissues (e.g., blood vessels) in the body. This type of 5D printed constructs not only have the shape-morphing ability but also provide controlled delivery of the embedded information (i.e., biomolecules in this study). Figure 9 illustrates the 5D printing process to obtain cell-laden constructs which are composed of a shape-morphing layer and a rat bone-marrow mesenchymal stem cell (rBMSC)-containing and biomolecule-delivering layer. The shape-morphing layer was 4D printed using shape memory polymer PDLLA-co-TMC. This polymer has a glass transition temperature at around 37 °C, which enables it to undergo 3D shape change upon heating from room temperature to the physiological temperature. In the current study, the printed PDLLA-co-TMC layer, which was made according to our established method [52], could transform from a 2D planar structure to a 3D curved shape by responding to heat. Briefly, as shown in Fig. 9, a 25% (w/v) PDLLA-co-TMC solution was prepared by dissolving the polymer in dichloromethane (DCM, Applied Biosystems, Ireland) and was then printed onto a glass slide coated with a thin layer of Vaseline to generate a 2D planar porous structure. The dried printed 2D structures were then reshaped into tubular structures using glass rods with different diameters at 80 °C for 90 min. Afterwards, the tubular structures with different diameters were cut and then flattened at 25 °C to take up the temporary 2D planar shape. As such, the shape-morphing layer of the constructs under construction was fabricated. The next step was to fabricate the rBMSC-containing and biomolecule-delivering layer (i.e., the information-embedded layer) using a dual-nozzle 3D printing system (3D Discovery™ Evolution, regenHU Ltd, Switzerland). In this step, two types of inks/bioinks were prepared. The first was an ink of 10% (w/v) GelMA (with a modification degree of about 50~60%) hydrogel containing two types of biomolecules: 200 ng/ml TGF-β1 (shortened as “TGF” hereafter) and 3 mg/ml ascorbic acid (AA). The second was a bioink prepared with 5% (w/v) gelatin (Gel) and 5% (w/v) GelMA (with a modification degree of about 20~30%) hydrogel blend containing rBMSCs (at 1 × 106 cell/ml). All hydrogels were sterilized by 60Co γ-ray irradiation before the addition of biomolecules or living cells. After the preparation of these two inks/bioinks, they were put into two separate syringes and used for printing alternately to form the information-embedded layer. rBMSC-laden structs were printed first using the bioink, and the ink containing biomolecules was printed into the space between two parallel rBMSC-laden structs. After printing, the information-embedded layer was exposed to a UV light at 365 nm wavelength and 365 mW power for 60 s to crosslink GelMA and Gel/GelMA hydrogels. Finally, the shape-morphing layer and information-embedded layer were combined to form bilayer scaffolds. When heated to 37 °C, the bilayer scaffolds should be able to self-bend to form curved structures as designed or self-fold to form tubular structures. With local delivery of the embedded information, i.e., the biomolecules, the bilayer scaffolds were expected to have rBMSCs induced to differentiate into smooth muscle cells (SMCs). As such, shape-morphing, rBMSC- and biomolecule-incorporated bilayer tissue engineering scaffolds had been made via 5D printing.
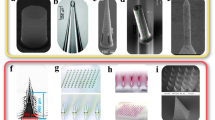
To confirm our design concepts and the realization of these concepts for demonstrating 5D printing in tissue engineering, additional tissue engineering scaffolds were made. Subsequently, various experiments were performed. PDLLA-co-TMC based single-layer scaffolds and bilayer scaffolds were fabricated for studying their morphology, structure and shape-morphing ability. Firstly, single-layer tubular PDLLA-co-TMC scaffolds with different diameters were made. As shown in Fig. 10(a), porous temporary 2D planar PDLLA-co-TMC scaffolds of different sizes were 4D printed. These temporary structures were planar at 25 °C when viewed from the top and side. After being heated to 37 °C via immersion in 37 °C water, they could automatically fold into tubular structures of different diameters. The shape-morphing process was quick and was completed within one minute, similar to what we had shown in a previous study [81]. Afterwards, bilayer scaffolds were fabricated where the first layer was a shape-morphing layer and the second layer was an information-embedded layer without cells, as shown in Fig. 10(b). The information-embedded layer was printed using GelMA-based hydrogels. It could be seen that the bilayer scaffolds remained in the temporary planar state at 25 °C when viewed from the top and side. They could also quickly complete the self-tubing process upon heating to above 37 °C, indicating that the addition of the second information-embedded layer did not affect the self-tubing ability of 4D printed PDLLA-co-TMC scaffolds, which was consistent with our previous study [52]. The structure and morphology of both types of scaffolds in the permanent tubular shape were examined using a field emission scanning electron microscope (Hitachi S3400N VP SEM, Japan), and SEM images are shown in Fig. 10(c). From the top view, it could be seen that both types of scaffolds exhibited the designed, regular macropores, demonstrating good structural features of the printed scaffolds. In addition, the GelMA-based hydrogel layer was seen through the regular macropores of the outer PDLLA-co-TMC layer in the bilayer scaffolds. From the side view, it could be seen that both types of scaffolds exhibited circular structures and the information-embedded layer of bilayer scaffolds was tightly attached to the PDLLA-co-TMC layer, suggesting successful fabrication of shape-morphing and information-embedded bilayer scaffolds via 5D printing.
4D/5D printed porous scaffolds. (a) Photographs showing top and side views of single-layer PDLLA-co-TMC scaffolds in the temporary planar state at 25 °C and in the permanent tubular shape at 37 °C with different diameters; (b) photographs showing top and side views of PDLLA-co-TMC/Gel-GelMA bilayer scaffolds in the temporary planar state at 25 °C and in the permanent tubular shape at 37 °C with different diameters; (c) SEM images providing top and side views of printed scaffolds in the permanent tubular shape (Scale bar: 5 mm).
PDLLA-co-TMC is an amorphous shape memory polymer. Depending on the PDLLA to TMC ratio in the polymer, it exhibits different glass transition temperatures and mechanical properties. In this 5D printing demonstration study, PDLLA-co-TMC with a PDLLA to TMC ratio of 9:1 was used. At this ratio, PDLLA-co-TMC has a glass transition temperature (Tg) slightly higher than human body temperature of 37 °C. This characteristic of this PDLLA-co-TMC polymer allows our 5D printed scaffolds to stay in the temporary planar state stably at 25 °C and then transform to the tubular shape upon heating to the body temperature according to our programmed design. In the current study, the self-tubing process of printed scaffolds had resulted from the temperature gradient within the PDLLA-co-TMC scaffold layer caused by the reshaping process at 80 °C. Such temperature gradient, from the surface to the interior, of PDLLA-co-TMC scaffolds, could affect the degree of molecular orientation in PDLLA-co-TMC, leading to the self-folding ability of PDLLA-co-TMC scaffolds or PDLLA-co-TMC scaffold layer in complex scaffold/constructs. Finally, the ability of self-folding into tubular structures of bilayer scaffolds was well noted even though the PDLLA-co-TMC shape-morphing layer was joined by an information-embedded layer in the bilayer scaffolds. This observation indicated well the desired shape-morphing property of 5D printed bilayer scaffolds.
To confirm the role/effects of the fifth dimension, i.e., information, in 5D printed structures, experiments were conducted on 5D printing of cell-laden and biomolecule-delivery constructs and on the effects of in situ delivered information (i.e., biomolecules) on rBMSC behaviour. Firstly, bioprinting of the rBMSC-containing Gel/GelMA bioink was performed to investigate and optimize cell state immediately after bioprinting and after in vitro culture of bioprinted structures for 1 and 3 days. The viability of rBMSCs incorporated in Gel/GelMA hydrogel scaffolds was studied using a LIVE/DEAD assay and the results are shown in Fig. 11. Using this assay, living cells were stained by calcein AM and would show green fluorescence under a fluorescence microscope, while dead cells were stained by ethidium homodimer EthD-1 and would show red fluorescence. From the day 0 result (i.e., immediately after bioprinting), it could be observed that most cells survived the bioprinting process even though some dead cells could be seen. The cell death was caused mainly by the exposure to the UV light and by the occurrence of radical polymerization of GelMA, as was explained by other researchers according to their similar investigations [128]. Furthermore, cells in the hydrogel at this time point stayed in the rounded shape, which was commonly observed in bioprinting studies of cell-laden hydrogels [34, 66, 101, 102]. After 1-day in vitro culture, interestingly, it was noted that most cells had been released from the hydrogel and that nearly no dead cells could be seen. It could be speculated that rBMSCs came out of the Gel/GelMA hydrogel owing to biodegradation of the hydrogel. The bioink was composed of rBMSCs, gelatin and lowly modified GelMA together with a low concentration of photoinitiator (2-hydroxy-2-methylpropiophenone, ~ 0.1% (v/v)). Such hydrogels after UV crosslinking of about 60 s (UV light intensity: 360 mW) could degrade fairly quickly when cultured at 37 °C. In addition, from the 10 × magnification images, the released rBMSCs could be seen to attach to the substrate and became spreading, which were very good for the subsequent cell differentiation under the influence of locally delivered biomolecules, i.e., the information. After 3-day in vitro culture, the released and adhering rBMSCs grew well and exhibited good proliferation, suggesting the good bioprinting part of 5D printing in the current study, as well as good fabrication of rBMSC-laden structs in the information-embedded layer of 5D bioprinted constructs.
To investigate the role/influence of the 5th dimension (i.e., embedded information, with its in situ delivery) on the incorporated living cells in 5D printed constructs, three types of the information layer containing different biomolecules, i.e., information, were fabricated by printing 10% (w/v) GelMA hydrogels containing AA, TGF, or TGF + AA (shortened as “TA” hereafter). GelMA hydrogel without biomolecules was also printed and set as the control. The printed information layers were UV-crosslinked for 60 s to stabilize the structure. In inks for the information layer, pure GelMA hydrogel with modified substitution of about 50%~60% was used so that the printed structures could stay stable and maintain a controlled and sustained delivery of the information (i.e., biomolecules). The bioprinted information layer scaffolds were cultured under the normal condition at 37 °C and after 5-day culture, the expression of F-actin and SMC-specific proteins (i.e., α-SMA and CALP) was studied to reveal the effects of locally delivered information (i.e., biomolecules) on rBMSCs, and the results are shown in Fig. 12. Abundant F-actin (red colored) together with cell nucleus (blue colored) was seen for cells in all three types of scaffolds. However, rBMSCs cultured in information-free scaffolds (the first column on the left in Fig. 12) showed relatively smaller and narrower cytoskeleton and lower cell density in comparison with those in information-embedded scaffolds (the AA, TGF and TA columns in Fig. 12). These results have demonstrated that the released embedded-information could improve the proliferation and cytoskeleton development of rBMSCs. Furthermore, as required by the definition of 5D printing and hence important for the cell-laden constructs manufactured, it was also shown that while no SMC-specific protein molecules were seen in the control group, cells in scaffolds embedded with AA or TGF displayed expressions of SMC-specific markers, i.e., α-smooth muscle actin (α-SMA) and h1-calponin (CALP). These results suggested that AA or TGF biomolecules alone were able to induce the differentiation of rBMSCs towards SMCs, which was in agreement with studies conducted by others [129, 130]. In addition, it could be seen that rBMSCs in scaffolds containing both TGF and AA exhibited significantly higher expression levels of α-SMA and CALP than scaffolds containing TGF or AA alone. This result indicated that TGF and AA had a synergetic effect on the differentiation of rBMSCs into SMCs, which was also observed in Narita et al.’s study [15]. Therefore, it is shown that the local delivery of information (TGF and AA), i.e., the 5th dimension in 5D printed structures, could affect the local environment for rBMSCs and cause the differentiation of rBMSCs towards SMCs, fullfilling what 5D printing aimed at in the current study. In the context of tissue engineering, these results have shown the importance and significant effects of incoporating apporriate biomolecules (including growth factors) in advanced MSC-laden constructs for guilding/controlling the cell hevhaior.
In human history, printing technologies, from 2D to 3D to 4D printing, play very important roles in various aspects of social development, including information assembly and dissemination, education, religion, manufacturing, etc. Over the past 30 years, 3D printing, since its first appearance in 1986, has made astonishing achievements in different areas and industries, including product design and development, consumer goods, transportation, healthcare, environmental protection, science, and education. Tissue engineering has greatly benefited from 3D printing, which has significantly expanded our ability to fabricate complex and advanced tissue engineering products with great precision, controlled architectures, and enhanced properties for regenerating different body tissues and organs. Applications of 3D printing for different tissues and organs (skin, vasculature, bone, articular cartilage, etc.) are now commonly seen and frequently reported. Over the past 10 years, 4D printing, since its introduction in 2013, has gained increasing attention in different areas such as soft robotics, smart sensors, and biomedical devices. Also, 4D printing in tissue engineering has been increasingly explored for, for example, fabricating self-tubing structures for blood vessel repair and for making shape-morphing bone scaffolds to match the defect shape and size after scaffold deployment. The progress in 3D and 4D printing has now enabled additive manufacturing to move forward into 5D printing which uses embedded information as the fifth dimension, in addition to 3D space and time. Just like 3D and 4D printing, 5D printing with the added information dimension will have great influences in different fields, such as biomedical engineering, of which tissue engineering is an essential part. For tissue engineering, in this article, we have shown a demonstration study for 5D printing which fabricated new bilayer scaffolds with both shape-morphing ability and controlled delivery of information (i.e., incorporated biomolecules). The 5D printed scaffolds could automatically fold up by responding to heating to the body temperature and the embedded and control-delivered information could change the environment of locally released living MSCs and induce the MSCs to differentiate into SMCs, which will be highly useful for the regeneration of tubular tissues such as vasculature and gastrointestinal tract. This demonstration study has also indicated the great potential of 5D printing in fabricating advanced scaffolds or constructs for regenerating complex human body tissues and organs.
5D printing is still at its very early stage of development and faces several major challenges. Firstly, it has been conceptualized only recently and is not yet widely known among researchers. Secondly, the information used in 5D printing to cause changes in the environment is currently very limited and requires further developments in the research community. For 5D printing in tissue engineering, apart from growth factors that were used in the demonstration study and presented in this article, other cell signals/messages (i.e., some proteins or molecules produced by cells) as potential, usable information should be investigated. These cell messages can be incorporated into 5D printed structures as the fifth dimension in AM and will be sent to the target tissues after deployment of the 5D printed structures, followed by changing the environment and triggering the changes of cells. Thirdly, currently available polymers suitable for 5D printing (Table S8) are very limited and hence new or novel biomaterials for 5D printing should be researched extensively and developed. Fourthly, there are still very few studies on 5D printing, and hence more research is needed to explore and realize its potential. Finally, the application of 5D printing in tissue engineering is not often reported and in this regard, more research is needed to see the benefits and potential. Nevertheless, as a new development of additive manufacturing, 5D printing can find many promising applications in translational clinical research and practices. 5D bioprinted cell-laden structures with embedded information and dynamic features will better recapitulate native tissues and organs, which can be used to gain deeper understanding of cell signaling pathways in basic research. Also, 5D printed information-embedded tissue engineering scaffolds will provide improved performance and hence can speed up tissue regeneration, leading to improved clinical outcomes. Furthermore, 5D printing can be used to produce personalized products with customized information and geometry for individual patients, leading to further developments in precision medicine.
Concluding remarks
This mini-review and prospective article has provided a concise and up-to-date review of recent advances in 3D/4D printing and bioprinting in tissue engineering. Current major 3D/4D printing technologies usable for tissue engineering and also biomaterials processable by 3D printing technologies for tissue engineering applications are presented and discussed. Applications of 3D/4D printing and bioprinting in tissue engineering for obtaining different static and dynamic scaffolds or cell-scaffold constructs for regenerating body tissues and organs, from relatively simple skin to very complex heart, are provided and analyzed. Finally, the concept of 5D printing is presented and explained, and using tissue engineering, the practice of 5D printing is demonstrated. The information embedded in 5D printed tissue engineering scaffolds and cell-scaffold constructs can be biomolecules such as GFs. The demonstration study has showed successful fabrication via 5D printing of shape-morphing, MSC-containing and biomolecule-delivering bilayer tissue engineering scaffolds. Such bilayer scaffolds can not only change from 2D planar shape to 3D tubular structures at the human body temperature but also deliver the embedded information to induce the incorporated rBMSCs to proliferate and differentiate for regenerating the target tissue, demonstrating the power of 5D printing for tackling challenges in regenerating complex body tissues. Moving from 3D/4D printing to 5D printing, AM goes forward from making passive/inactive products to producing active/intelligent structures that can cause intended, positive changes in the environment. 5D printing opens a new arena for the development of additive manufacturing and in the tissue engineering area, leading to the fabrication of novel tissue engineering scaffolds with high clinical performance for the regeneration of different human tissues and organs.
References
J. Lai, C. Wang, M. Wang, 3D printing in biomedical engineering: Processes, materials, and applications. Appl. Phys. Rev. 8(2), 021322 (2021)
S. Zhao, M. Tayyebi, M. Yarigarravesh, G. Hu, A review of magnesium corrosion in bio-applications: mechanism, classification, modeling, in-vitro, and in-vivo experimental testing, and tailoring Mg corrosion rate. J. Mater. Sci. 58(30), 12158 (2023)
C. Wang, W. Huang, Y. Zhou, L. He, Z. He, Z. Chen, X. He, S. Tian, J. Liao, B. Lu, Y. Wei, M. Wang, 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 5(1), 82 (2020)
K. Xie, N. Wang, Y. Guo, S. Zhao, J. Tan, L. Wang, G. Li, J. Wu, Y. Yang, W. Xu, J. Chen, W. Jiang, P. Fu, Y. Hao, Additively manufactured biodegradable porous magnesium implants for elimination of implant-related infections: an in vitro and in vivo study. Bioact. Mater. 8, 140 (2022)
S.V. Murphy, A. Atala, 3D bioprinting of tissues and organs. Nat. Biotechnol. 32(8), 773 (2014)
C. Gao, Y. Li, X. Liu, J. Huang, Z. Zhang, 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem. Eng. J. 451, 138788 (2023)
X. Kuang, D.J. Roach, J. Wu, C.M. Hamel, Z. Ding, T. Wang, M.L. Dunn, H.J. Qi, Advances in 4D Printing: materials and applications. Adv. Funct. Mater. 29(2), 1805290 (2019)
D. Rahmatabadi, M. Aberoumand, K. Soltanmohammadi, E. Soleyman, I. Ghasemi, M. Baniassadi, K. Abrinia, A. Zolfagharian, M. Bodaghi, M. Baghani, A new strategy for achieving shape memory effects in 4D printed two-layer composite structures. Polymers 14(24), 5446 (2022)
M. Aberoumand, K. Soltanmohammadi, D. Rahmatabadi, E. Soleyman, I. Ghasemi, M. Baniassadi, K. Abrinia, M. Bodaghi, M. Baghani, 4D Printing of polyvinyl chloride (PVC): a detailed analysis of microstructure, programming, and shape memory performance. Macromol. Mater. Eng. 308(7), 2200677 (2023)
M. Wang: 5D Printing and Its Application in Tissue Engineering, in Materials Research Society (MRS) Spring Meeting, (Materials Virtual Research Society, 2021).
C.K. Chua, W.Y. Yeong, Bioprinting: Principles and Applications (World Scientific Publishing Co Inc, Singapore, 2014)
J.P. Vacanti, R. Langer, Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354, S32 (1999)
C. Wang, Q. Zhao, M. Wang, Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 9(2), 025031 (2017)
B. Duan, M. Wang, W.Y. Zhou, W.L. Cheung, Z.Y. Li, W.W. Lu, Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 6(12), 4495 (2010)
Y. Narita, A. Yamawaki, H. Kagami, M. Ueda, Y. Ueda, Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res. 333(3), 449 (2008)
G. Jiang, S. Li, K. Yu, B. He, J. Hong, T. Xu, J. Meng, C. Ye, Y. Chen, Z. Shi, G. Feng, W. Chen, S. Yan, Y. He, R. Yan, A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 128, 150 (2021)
W. Zhang, W. Shi, S. Wu, M. Kuss, X. Jiang, J.B. Untrauer, S.P. Reid, B. Duan, 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 12(3), 035020 (2020)
S. Bose, A. Bhattacharjee, D. Banerjee, A.R. Boccaccini, A. Bandyopadhyay, Influence of random and designed porosities on 3D printed tricalcium phosphate-bioactive glass scaffolds. Add. Manuf. 40, 101895 (2021)
M. Zhang, R. Lin, X. Wang, J. Xue, C. Deng, C. Feng, H. Zhuang, J. Ma, C. Qin, L. Wan, 3D printing of Haversian bone–mimicking scaffolds for multicellular delivery in bone regeneration. Sci. Adv. 6(12), 6725 (2020)
L. Zhu, H. Liang, F. Lv, D. Xie, C. Wang, Y. Mao, Y. Yang, Z. Tian, L. Shen, Design and compressive fatigue properties of irregular porous scaffolds for orthopedics fabricated using selective laser melting. ACS Biomater. Sci. Eng. 7(4), 1663 (2021)
J. Lai, C. Wang, J. Liu, S. Chen, C. Liu, X. Huang, J. Wu, Y. Pan, Y. Xie, M. Wang, Low temperature hybrid 3D printing of hierarchically porous bone tissue engineering scaffolds with in situ delivery of osteogenic peptide and mesenchymal stem cells. Biofabrication 14(4), 045006 (2022)
S. Li, W. Wang, W. Li, M. Xie, C. Deng, X. Sun, C. Wang, Y. Liu, G. Shi, Y. Xu, X. Ma, J. Wang, Fabrication of thermoresponsive hydrogel scaffolds with engineered microscale vasculatures. Adv. Funct. Mater. 31(27), 2102685 (2021)
A.E. Alexander, N. Wake, L. Chepelev, P. Brantner, J. Ryan, K.C. Wang, A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 7(1), 8 (2021)
Z.X. Khoo, J.E.M. Teoh, Y. Liu, C.K. Chua, S. Yang, J. An, K.F. Leong, W.Y. Yeong, 3D printing of smart materials: a review on recent progresses in 4D printing. Virt. Phys. Prototyp. 10(3), 103 (2015)
J. Lai, X. Ye, J. Liu, C. Wang, J. Li, X. Wang, M. Ma, M. Wang, 4D printing of highly printable and shape morphing hydrogels composed of alginate and methylcellulose. Mater. Des. 205, 109699 (2021)
T. van Manen, S. Janbaz, A.A. Zadpoor, Programming 2D/3D shape-shifting with hobbyist 3D printers. Mater. Horiz. 4(6), 1064 (2017)
A.C. Weems, M.C. Arno, W. Yu, R.T.R. Huckstepp, A.P. Dove, 4D polycarbonates via stereolithography as scaffolds for soft tissue repair. Nat. Commun. 12(1), 3771 (2021)
S.H. Kim, Y.B. Seo, Y.K. Yeon, Y.J. Lee, H.S. Park, M.T. Sultan, J.M. Lee, J.S. Lee, O.J. Lee, H. Hong, H. Lee, O. Ajiteru, Y.J. Suh, S.-H. Song, K.-H. Lee, C.H. Park, 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 260, 120281 (2020)
Y. Zheng, Q. Han, D. Li, F. Sheng, Z. Song, J. Wang, Promotion of tendon growth into implant through pore-size design of a Ti-6Al-4V porous scaffold prepared by 3D printing. Mater. Des. 197, 109219 (2021)
X. Li, B. Liu, B. Pei, J. Chen, D. Zhou, J. Peng, X. Zhang, W. Jia, T. Xu, Inkjet bioprinting of biomaterials. Chem. Rev. 120(19), 10793 (2020)
J. Zhang, P. Byers, A. Erben, C. Frank, L. Schulte-Spechtel, M. Heymann, D. Docheva, H.P. Huber, S. Sudhop, H. Clausen-Schaumann, Single cell bioprinting with ultrashort laser pulses. Adv. Funct. Mater. 31(19), 2100066 (2021)
L.K. Shopperly, J. Spinnen, J.-P. Krüger, M. Endres, M. Sittinger, T. Lam, L. Kloke, T. Dehne, Blends of gelatin and hyaluronic acid stratified by stereolithographic bioprinting approximate cartilaginous matrix gradients. J. Biomed. Mater. Res. Part B 110(10), 2310 (2022)
S. You, Y. Xiang, H.H. Hwang, D.B. Berry, W. Kiratitanaporn, J. Guan, E. Yao, M. Tang, Z. Zhong, X. Ma, D. Wangpraseurt, Y. Sun, T. Lu, S. Chen, High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 9(8), 7923 (2023)
B. Duan, L.A. Hockaday, K.H. Kang, J.T. Butcher, 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 101(5), 1255 (2013)
A. Gregor, E. Filová, M. Novák, J. Kronek, H. Chlup, M. Buzgo, V. Blahnová, V. Lukášová, M. Bartoš, A. Nečas, J. Hošek, Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 11, 31 (2017)
C. Wang, J. Lai, K. Li, S. Zhu, B. Lu, J. Liu, Y. Tang and Y. Wei: Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization Bioactive Materials. 6(1), 137 (2021).
W. Zhu, H. Cui, B. Boualam, F. Masood, E. Flynn, R.D. Rao, Z.-Y. Zhang, L.G. Zhang, 3d bioprinting mesenchymal stem cell-laden construct with core–shell nanospheres for cartilage tissue engineering. Nanotechnology 29(18), 185101 (2018)
H. Hong, Y.B. Seo, J.S. Lee, Y.J. Lee, H. Lee, O. Ajiteru, M.T. Sultan, O.J. Lee, S.H. Kim, C.H. Park, Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 232, 119679 (2020)
X. Jiang, S. Wu, M. Kuss, Y. Kong, W. Shi, P.N. Streubel, T. Li, B. Duan, 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact. Mater. 5(3), 636 (2020)
A.B.R. Touré, E. Mele, J.K. Christie, Multi-layer scaffolds of poly(caprolactone), poly(glycerol sebacate) and bioactive glasses manufactured by combined 3d printing and electrospinning. Nanomaterials 10(4), 89 (2020)
S.C. Millik, A.M. Dostie, D.G. Karis, P.T. Smith, M. McKenna, N. Chan, C.D. Curtis, E. Nance, A.B. Theberge, A. Nelson, 3D printed coaxial nozzles for the extrusion of hydrogel tubes toward modeling vascular endothelium. Biofabrication 11(4), 045009 (2019)
Y. Wang, R.K. Kankala, K. Zhu, S.-B. Wang, Y.S. Zhang, A.-Z. Chen, Coaxial extrusion of tubular tissue constructs using a gelatin/GelMA blend bioink. ACS Biomater. Sci. Eng. 5(10), 5514 (2019)
B. Grigoryan, S.J. Paulsen, D.C. Corbett, D.W. Sazer, C.L. Fortin, A.J. Zaita, P.T. Greenfield, N.J. Calafat, J.P. Gounley, A.H. Ta, F. Johansson, A. Randles, J.E. Rosenkrantz, J.D. Louis-Rosenberg, P.A. Galie, K.R. Stevens, J.S. Miller, Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364(6439), 458 (2019)
K. Peng, X. Liu, H. Zhao, H. Lu, F. Lv, L. Liu, Y. Huang, S. Wang, Q. Gu, 3D bioprinting of reinforced vessels by dual-cross-linked biocompatible hydrogels. ACS Appl. Bio Mater. 4(5), 4549 (2021)
S.L. Sing, J. An, W.Y. Yeong, F.E. Wiria, Laser and electron-beam powder-bed additive manufacturing of metallic implants: a review on processes, materials and designs. J. Orthop. Res. 34(3), 369 (2016)
A. Bandyopadhyay, B.V. Krishna, W. Xue, S. Bose, Application of laser engineered net shaping (LENS) to manufacture porous and functionally graded structures for load bearing implants. J. Mater. Sci. 20(1), 29 (2009)
J. Yang, H. Cai, J. Lv, K. Zhang, H. Leng, C. Sun, Z. Wang, Z. Liu, In vivo study of a self-stabilizing artificial vertebral body fabricated by electron beam melting. Spine 39(8), E486 (2014)
C. Wang, X. Ye, Y. Zhao, L. Bai, Z. He, Q. Tong, X. Xie, H. Zhu, D. Cai, Y. Zhou, B. Lu, Y. Wei, L. Mei, D. Xie, M. Wang, Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication 12(3), 035004 (2020)
C. Wang, H. Yue, W. Huang, X. Lin, X. Xie, Z. He, X. He, S. Liu, L. Bai, B. Lu, Y. Wei, M. Wang, Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 12(2), 025030 (2020)
M. Wang, Q. Zhao, Biomedical composites. Ref. Module Biomed. Sci. 45, 78 (2019)
X. Li, B. Cho, R. Martin, M. Seu, C. Zhang, Z. Zhou, S.C. Ji, X. Jiang, L. Chen, G. Walia, J. Yan, M. Callanan, H. Liu, K. Colbert, J. Morrissette-mcalmon, W. Grayson, S. Reddy, M.S. Justin, H.-Q. Mao, Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 11(490), 6210 (2019)
J. Lai, J. Li, M. Wang, 3D Printed porous tissue engineering scaffolds with the self-folding ability and controlled release of growth factor. MRS Commun. 10(4), 579 (2020)
C. Wang, H. Yue, J. Liu, Q. Zhao, Z. He, K. Li, B. Lu, W. Huang, Y. Wei, Y. Tang, M. Wang, Advanced reconfigurable scaffolds fabricated by 4D printing for treating critical-size bone defects of irregular shapes. Biofabrication 12(4), 045025 (2020)
A. Kirillova, R. Maxson, G. Stoychev, C.T. Gomillion, L. Ionov, 4D biofabrication using shape-morphing hydrogels. Adv. Mater. 29(46), 1703443 (2017)
L. Zhang, Y. Xiang, H. Zhang, L. Cheng, X. Mao, N. An, L. Zhang, J. Zhou, L. Deng, Y. Zhang, A biomimetic 3D-self-forming approach for microvascular scaffolds. Adv. Sci. 7(9), 1903553 (2020)
Y.D. Zhao, J.H. Lai, M. Wang, 4D printing of self-folding hydrogel tubes for potential tissue engineering applications. Nano LIFE 11(04), 2141001 (2021)
X. Du, H. Cui, Q. Zhao, J. Wang, H. Chen, Y. Wang, Inside-Out 3D Reversible ion-triggered shape-morphing hydrogels. Research 2019, 6398296 (2019)
A.S. Gladman, E.A. Matsumoto, R.G. Nuzzo, L. Mahadevan, J.A. Lewis, Biomimetic 4D printing. Nat. Mater. 15(4), 413 (2016)
H. Wei, Q. Zhang, Y. Yao, L. Liu, Y. Liu, J. Leng, Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interf. 9(1), 876 (2017)
M. Hospodiuk, M. Dey, D. Sosnoski, I.T. Ozbolat, The bioink: a comprehensive review on bioprintable materials. Biotechnol. Adv. 35(2), 217 (2017)
B. Duan, E. Kapetanovic, L.A. Hockaday, J.T. Butcher, Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 10(5), 1836 (2014)
Y.-J. Choi, T.G. Kim, J. Jeong, H.-G. Yi, J.W. Park, W. Hwang, D.-W. Cho, 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv. Healthc. Mater. 5(20), 2636 (2016)
N.E. Fedorovich, J.R. De Wijn, A.J. Verbout, J. Alblas, W.J. Dhert, Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng. Part A 14(1), 127 (2008)
T. Jain, H.B. Baker, A. Gipsov, J.P. Fisher, A. Joy, D.S. Kaplan, I. Isayeva, Impact of cell density on the bioprinting of gelatin methacrylate (GelMA) bioinks. Bioprinting. 22, 00131 (2021)
L. Ouyang, J.P.K. Armstrong, Q. Chen, Y. Lin, M.M. Stevens, Void-free 3D bioprinting for in situ endothelialization and microfluidic perfusion. Adv. Funct. Mater. 30(1), 1908349 (2020)
Y. Wu, A. Wenger, H. Golzar, X. Tang, 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink. Sci. Rep. 10(1), 20648 (2020)
W. Ye, H. Li, K. Yu, C. Xie, P. Wang, Y. Zheng, P. Zhang, J. Xiu, Y. Yang, F. Zhang, Y. He, Q. Gao, 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 192, 108757 (2020)
J. Gong, C.C.L. Schuurmans, A.M.V. Genderen, X. Cao, W. Li, F. Cheng, J.J. He, A. López, V. Huerta, J. Manríquez, R. Li, H. Li, C. Delavaux, S. Sebastian, P.E. Capendale, H. Wang, J. Xie, M. Yu, R. Masereeuw, T. Vermonden, Y.S. Zhang, Complexation-induced resolution enhancement of 3D-printed hydrogel constructs. Nat. Commun. 11(1), 1267 (2020)
F. Zheng, B. Derby, J. Wong, Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 13(3), 035006 (2021)
B.S. Kim, G. Gao, J.Y. Kim, D.W. Cho, 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin. Adv. Healthcare Mater. 8(7), 1801019 (2019)
J. Zhang, S. Yun, A. Karami, B. Jing, A. Zannettino, Y. Du, H. Zhang, 3D printing of a thermosensitive hydrogel for skin tissue engineering: a proof of concept study. Bioprinting. 19, 00089 (2020)
J. Ma, C. Qin, J. Wu, H. Zhang, H. Zhuang, M. Zhang, Z. Zhang, L. Ma, X. Wang, B. Ma, J. Chang, C. Wu, 3D printing of strontium silicate microcylinder-containing multicellular biomaterial inks for vascularized skin regeneration. Adv. Healthc. Mater. 10(16), 2100523 (2021)
F. Zhou, Y. Hong, R. Liang, X. Zhang, Y. Liao, D. Jiang, J. Zhang, Z. Sheng, C. Xie, Z. Peng, X. Zhuang, V. Bunpetch, Y. Zou, W. Huang, Q. Zhang, E.V. Alakpa, S. Zhang, H. Ouyang, Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 258, 120287 (2020)
C.S. Shin, F.J. Cabrera, R. Lee, J. Kim, R.A. Veettil, M. Zaheer, A. Adumbumkulath, K. Mhatre, P.M. Ajayan, S.A. Curley, B.G. Scott, G. Acharya, 3D-bioprinted inflammation modulating polymer scaffolds for soft tissue repair. Adv. Mater. 33(4), 2003778 (2021)
N.R. Barros, H.-J. Kim, M.J. Gouidie, K. Lee, P. Bandaru, E.A. Banton, E. Sarikhani, W. Sun, S. Zhang, H.-J. Cho, M.C. Hartel, S. Ostrovidov, S. Ahadian, S.M. Hussain, N. Ashammakhi, M.R. Dokmeci, R.D. Herculano, J. Lee, A. Khademhosseini, Biofabrication of endothelial cell, dermal fibroblast, and multilayered keratinocyte layers for skin tissue engineering. Biofabrication 13(3), 035030 (2021)
H.R. Lee, J.A. Park, S. Kim, Y. Jo, D. Kang, S. Jung, 3D microextrusion-inkjet hybrid printing of structured human skin equivalents. Bioprinting. 22, e00143 (2021)
M.S. Chaudhry, A. Czekanski, In-situ bioprinting of skin: a review. Bioprinting 31, e00271 (2023)
H. Chen, X. Ma, T. Gao, W. Zhao, T. Xu, Z. Liu, Robot-assisted in situ bioprinting of gelatin methacrylate hydrogels with stem cells induces hair follicle-inclusive skin regeneration. Biomed. Pharmacother. 158, 114140 (2023)
Y. Yang, Q. Zhang, T. Xu, H. Zhang, M. Zhang, L. Lu, Y. Hao, J.H. Fuh, X. Zhao, Photocrosslinkable nanocomposite ink for printing strong, biodegradable and bioactive bone graft. Biomaterials 263, 120378 (2020)
S. Miao, W. Zhu, N.J. Castro, M. Nowicki, X. Zhou, H. Cui, J.P. Fisher, L.G. Zhang, 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 6, 27226 (2016)
C. Wang, Y. Zhou, M. Wang, In situ delivery of rhBMP-2 in surface porous shape memory scaffolds developed through cryogenic 3D plotting. Mater. Lett. 189, 140 (2017)
Y. Zhou, R. Qin, T. Chen, K. Zhang, J. Gui, 3D bioprinting modified autologous matrix-induced chondrogenesis(AMIC) technique for repair of cartilage defects. Mater. Des. 203, 109621 (2021)
W. Chen, Y. Xu, Y. Liu, Z. Wang, Y. Li, G. Jiang, X. Mo, G. Zhou, Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 179, 107886 (2019)
P. Apelgren, M. Amoroso, K. Säljö, A. Lindahl, C. Brantsing, L.S. Orrhult, K. Markstedt, P. Gatenholm, L. Kölby, Long-term in vivo integrity and safety of 3D-bioprinted cartilaginous constructs. J. Biomed. Mater. Res. Part B 109(1), 126 (2021)
M.R. Rad, F. Fahimipour, E. Dashtimoghadam, H. Nokhbatolfoghahaei, L. Tayebi, A. Khojasteh, Osteogenic differentiation of adipose-derived mesenchymal stem cells using 3D-Printed PDLLA/ β-TCP nanocomposite scaffolds. Bioprinting 21, 00117 (2021)
W. Chen, Y. Xu, Y. Li, L. Jia, X. Mo, G. Jiang, G. Zhou, 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration Chem. Eng. J. 382, 122986 (2020)
S. Chae, S.S. Lee, Y.J. Choi, D.H. Hong, G. Gao, J.H. Wang, D.W. Cho, 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 267, 120466 (2021)
S. Critchley, E.J. Sheehy, G. Cunniffe, P. Diaz-Payno, S.F. Carroll, O. Jeon, E. Alsberg, P.A. Brama, D.J. Kelly, 3D printing of fiber-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 113, 130 (2020)
Y. Meng, J. Cao, Y. Chen, Y. Yu, L. Ye, 3D printing of a poly (vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J. Mater. Chem. B 8(4), 677 (2020)
K. Markstedt, A. Mantas, I. Tournier, H.C.M. Ávila, D. Hägg, P. Gatenholm, 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromol 16(5), 1489 (2015)
L.K. Narayanan, P. Huebner, M.B. Fisher, J.T. Spang, B. Starly, R.A. Shirwaiker, 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater. Sci. Eng. 2(10), 1732 (2016)
H. Zhang, Z. Pei, C. Wang, M. Li, H. Zhang, J. Qu, Electrohydrodynamic 3D printing scaffolds for repair of achilles tendon defect in rats. Tissue Eng. Part A 27(19–20), 1343 (2021)
Y. Wu, Z. Wang, J.Y.H. Fuh, Y.S. Wong, W. Wang, E.S. Thian, Direct E-jet printing of three-dimensional fibrous scaffold for tendon tissue engineering. J. Biomed. Mater. Res. Part B 105(3), 616 (2017)
A.A. Pitaru, J.-G. Lacombe, M.E. Cooke, L. Beckman, T. Steffen, M.H. Weber, P.A. Martineau, D.H. Rosenzweig, Investigating commercial filaments for 3D printing of stiff and elastic constructs with ligament-like mechanics. Micromachines. 11(9), 846 (2020)
J.P. Quint, A. Mostafavi, Y. Endo, A. Panayi, C.S. Russell, A. Nourmahnad, C. Wiseman, L. Abbasi, M. Samandari, A. Sheikhi, K. Nuutila, I. Sinha, A. Tamayol, In vivo printing of nanoenabled scaffolds for the treatment of skeletal muscle injuries. Adv. Healthc. Mater. 10(10), 2002152 (2021)
J.H. Kim, I. Kim, Y.J. Seol, I.K. Ko, J.J. Yoo, A. Atala, S.J. Lee, Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 11(1), 1 (2020)
M.S. Kang, J.I. Kang, P. Le Thi, K.M. Park, S.W. Hong, Y.S. Choi, D.-W. Han, K.D. Park, Three-dimensional printable gelatin hydrogels incorporating graphene oxide to enable spontaneous myogenic differentiation. ACS Macro Lett. 10(4), 426 (2021)
G. Constante, I. Apsite, H. Alkhamis, M. Dulle, M. Schwarzer, A. Caspari, A. Synytska, S. Salehi, L. Ionov, 4D biofabrication using a combination of 3D printing and melt-electrowriting of shape-morphing polymers. ACS Appl. Mater. Interf. 13(11), 12767 (2021)
J. Uribe-Gomez, A. Posada-Murcia, A. Shukla, M. Ergin, G. Constante, I. Apsite, D. Martin, M. Schwarzer, A. Caspari, A. Synytska, S. Salehi, L. Ionov, Shape-Morphing Fibrous Hydrogel/Elastomer Bilayers Fabricated by a Combination of 3D Printing and Melt Electrowriting for Muscle Tissue Regeneration. ACS Appl. Bio Mater. 4(2), 1720 (2021)
Q. Liang, F. Gao, Z. Zeng, J. Yang, M. Wu, C. Gao, D. Cheng, H. Pan, W. Liu, C. Ruan, Coaxial scale-up printing of diameter-tunable biohybrid hydrogel microtubes with high strength perfusability, and endothelialization. Adv. Funct. Mater. 30(43), 2001485 (2020)
L. Shao, Q. Gao, C. Xie, J. Fu, M. Xiang, Y. He, Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication 12(3), 035014 (2020)
D.B. Kolesky, R.L. Truby, A.S. Gladman, T.A. Busbee, K.A. Homan, J.A. Lewis, 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 26(19), 3124 (2014)
B. Ren, K. Song, A.R. Sanikommu, Y. Chai, M.A. Longmire, W. Chai, W.L. Murfee, Y. Huang, Study of sacrificial ink-assisted embedded printing for 3D perfusable channel creation for biomedical applications. Appl. Phys. Rev. 9(1), 011408 (2022)
L. Shao, Q. Gao, C. Xie, J. Fu, M. Xiang, Y. He, Synchronous 3D bioprinting of large-scale cell-laden constructs with nutrient networks. Adv. Healthc. Mater. 9(15), 1901142 (2020)
C. Zhang, D. Cai, P. Liao, J.-W. Su, H. Deng, B. Vardhanabhuti, B.D. Ulery, S.Y. Chen, J. Lin, 4D Printing of shape-memory polymeric scaffolds for adaptive biomedical implantation. Acta Biomater. 122, 101 (2021)
K. Zeinali, M.T. Khorasani, A. Rashidi, M.D. Joupari, Preparation and characterization of graphene oxide aerogel/gelatin as a hybrid scaffold for application in nerve tissue engineering. Int. J. Polym. Mater. Polym. Biomat. 70(10), 674 (2021)
A.F. Girão, J. Sousa, A. Domínguez-Bajo, A. González-Mayorga, I. Bdikin, E. Pujades-Otero, N. Casañ-Pastor, M.J. Hortigüela, G. Otero-Irurueta, A. Completo, M.C. Serrano, P.A.A.P. Marques, 3D reduced graphene oxide scaffolds with a combinatorial fibrous-porous architecture for neural tissue engineering. ACS Appl. Mater. Interf. 12(35), 38962 (2020)
X. Liu, M. Hao, Z. Chen, T. Zhang, J. Huang, J. Dai, Z. Zhang, 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 272, 120771 (2021)
S. Miao, H. Cui, M. Nowicki, L. Xia, X. Zhou, S.J. Lee, W. Zhu, K. Sarkar, Z. Zhang, L.G. Zhang, Stereolithographic 4D bioprinting of multiresponsive architectures for neural engineering. Adv. Biosyst. 2(9), 1800101 (2018)
Z. Zhang, M.L. Jørgensen, Z. Wang, J. Amagat, Y. Wang, Q. Li, M. Dong, M. Chen, 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials 253, 120108 (2020)
http://gco.iarc.fr/today/home. Accessed date: June 4 2023.
S. Moon, S.K. Hasan, Y.S. Song, F. Xu, H.O. Keles, F. Manzur, S. Mikkilineni, J.W. Hong, J. Nagatomi, E. Haeggstrom, Layer by layer three-dimensional tissue epitaxy by cell-laden hydrogel droplets. Tissue Eng. Part C 16(1), 157 (2009)
C.Y. Wang, H. Wang, Q.P. Guo, X. Ang, B. Li, F.X. Han, Y.X. Fu, W.G. Chen, Bladder muscle regeneration enhanced by sustainable delivery of heparin from bilayer scaffolds carrying stem cells in a rat bladder partial cystectomy model. Biomed. Mater. 16(3), 035033 (2021)
Q. Mao, Y. Wang, Y. Li, S. Juengpanich, W. Li, M. Chen, J. Yin, J. Fu, X. Cai, Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater. Sci. Eng. C 109, 110625 (2020)
X. Ma, X. Qu, W. Zhu, Y.-S. Li, S. Yuan, H. Zhang, J. Liu, P. Wang, C.S.E. Lai, F. Zanella, G.-S. Feng, F. Sheikh, S. Chien, S. Chen, Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. 113(8), 2206 (2016)
D. Kang, G. Hong, S. An, I. Jang, W.-S. Yun, J.H. Shim, S. Jin, Bioprinting of multiscaled hepatic lobules within a highly vascularized construct. Small 16(13), 1905505 (2020)
G. Janani, S. Priya, S. Dey, B.B. Mandal, Mimicking native liver lobule microarchitecture in vitro with parenchymal and non-parenchymal cells using 3D bioprinting for drug toxicity and drug screening applications. ACS Appl. Mater. Interf. 14(8), 10167 (2022)
J. Li, J. Chu, V.C. Lui, S. Chen, Y. Chen, P.K. Tam, Bioengineering liver organoids for diseases modelling and transplantation. Bioengineering 9(12), 796 (2022)
H. Yang, L. Sun, Y. Pang, D. Hu, H. Xu, S. Mao, W. Peng, Y. Wang, Y. Xu, Y.-C. Zheng, S. Du, H. Zhao, T. Chi, X. Lu, X. Sang, S. Zhong, X. Wang, H. Zhang, P. Huang, W. Sun, Y. Mao, Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 70(3), 567 (2021)
A. Lee, A.R. Hudson, D.J. Shiwarski, J.W. Tashman, T.J. Hinton, S. Yerneni, J.M. Bliley, P.G. Campbell, A.W. Feinberg, 3D bioprinting of collagen to rebuild components of the human heart. Science 365(6452), 482 (2019)
N. Noor, A. Shapira, R. Edri, I. Gal, L. Wertheim, T. Dvir, 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 6(11), 1900344 (2019)
E. Mirdamadi, J.W. Tashman, D.J. Shiwarski, R.N. Palchesko, A.W. Feinberg, FRESH 3D bioprinting a full-size model of the human heart. ACS Biomat. Sci. Eng. 6(11), 6453 (2020)
Y. Wang, H. Cui, Y. Wang, C. Xu, T.J. Esworthy, S.Y. Hann, M. Boehm, Y.-L. Shen, D. Mei, L.G. Zhang, 4D printed cardiac construct with aligned myofibers and adjustable curvature for myocardial regeneration. ACS Appl. Mater. Interf. 13(11), 12746 (2021)
B. Kong, Y. Chen, R. Liu, X. Liu, C. Liu, Z. Shao, L. Xiong, X. Liu, W. Sun, S. Mi, Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 11(1), 1435 (2020)
J.A. Brassard, M. Nikolaev, T. Hübscher, M. Hofer, M.P. Lutolf, Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 20(1), 22 (2020)
M. Nikolaev, O. Mitrofanova, N. Broguiere, S. Geraldo, D. Dutta, Y. Tabata, B. Elci, N. Brandenberg, I. Kolotuev, N. Gjorevski, H. Clevers, M.P. Lutolf, Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585(7826), 574 (2020)
K.T. Lawlor, J.M. Vanslambrouck, J.W. Higgins, A. Chambon, K. Bishard, D. Arndt, P.X. Er, S.B. Wilson, S.E. Howden, K.S. Tan, F. Li, L.J. Hale, B. Shepherd, S. Pentoney, S.C. Presnell, A.E. Chen, M.H. Little, Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20(2), 260–271 (2020)
K.S. Lim, J.H. Galarraga, X. Cui, G.C. Lindberg, J.A. Burdick, T.B. Woodfield, Fundamentals and applications of photo-cross-linking in bioprinting. Chem. Rev. 120(19), 10662 (2020)
K.M. Choi, Y.K. Seo, H.-H. Yoon, K.Y. Song, S.Y. Kwon, H.S. Lee, J.-K. Park, Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 105(6), 586 (2008)
M. Floren, W. Bonani, A. Dharmarajan, A. Motta, C. Migliaresi, W. Tan, Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 31, 156 (2016)
S. Romanazzo, T.G. Molley, S. Nemec, K. Lin, R. Sheikh, J.J. Gooding, B. Wan, Q. Li, K.A. Kilian, I. Roohani, Synthetic bone-like structures through omnidirectional ceramic bioprinting in cell suspensions. Adv. Funct. Mater. 31(13), 2008216 (2021)
S. Lee, E.S. Sani, A.R. Spencer, Y. Guan, A.S. Weiss, N. Annabi, Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 32(45), 2003915 (2020)
Y. Li, S. Lv, H. Yuan, G. Ye, W. Mu, Y. Fu, X. Zhang, Z. Feng, Y. He, W. Chen, Peripheral nerve regeneration with 3D printed bionic scaffolds loading neural crest stem cell derived schwann cell progenitors. Adv. Funct. Mater. 31(16), 2010215 (2021)
W.L. Ng, J.T.Z. Qi, W.Y. Yeong, M.W. Naing, Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 10(2), 025005 (2018)
R. da Conceicao, D. Pal, A.M. Ferreira, P. Gentile, M. Benning, K. Dalgarno, Reactive jet impingement bioprinting of high cell density gels for bone microtissue fabrication. Biofabrication 11(1), 015014 (2018)
J. Schöneberg, F. De Lorenzi, B. Theek, A. Blaeser, D. Rommel, A.J.C. Kuehne, F. Kießling, H. Fischer, Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 8(1), 10430 (2018)
D. Hakobyan, O. Kerouredan, M. Remy, N. Dusserre, C. Medina, R. Devillard, J.-C. Fricain and H. Oliveira: Laser-Assisted Bioprinting for Bone Repair, in 3D Bioprinting: Principles and Protocols, edited by J. M. Crook (Springer US, 2020), pp. 135.
R.D. Ventura, An overview of laser-assisted bioprinting (LAB) in tissue engineering applications. Med. Lasers. 10(2), 76 (2021)
Y.W. Chen, H.Y. Fang, M.Y. Shie, Y.F. Shen, The mussel-inspired assisted apatite mineralized on PolyJet material for artificial bone scaffold. Int. J. Bioprint. 5(2), 197 (2019)
P. Heinl, L. Müller, C. Körner, R.F. Singer, F.A. Müller, Cellular Ti–6Al–4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 4(5), 1536 (2008)
Y. Zhang, T. Lei, C. Tang, Y. Chen, Y. Liao, W. Ju, H. Zhang, B. Zhou, R. Liang, T. Zhang, C. Fan, X. Chen, Y. Zhao, Y. Xie, J. Ye, B.C. Heng, X. Chen, Y. Hong, W. Shen, Z. Yin, 3D printing of chemical-empowered tendon stem/progenitor cells for functional tissue repair. Biomaterials 271, 120722 (2021)
C. Li, M. Kuss, Y. Kong, F. Nie, X. Liu, B. Liu, A. Dunaevsky, P. Fayad, B. Duan, X. Li, 3D printed hydrogels with aligned microchannels to guide neural stem cell migration. ACS Biomat. Sci. Eng. 7(2), 690 (2021)
Y. Wang, S. Chen, H. Liang, Y. Liu, J. Bai, M. Wang, Digital light processing (DLP) of nano biphasic calcium phosphate bioceramic for making bone tissue engineering scaffolds. Ceram. Int. 48(19), 27681 (2022)
H. Xiang, X. Dai, W. Xu, S. Li, X. Yang, Z. Huang, R. Li, C. Yang, H. Chang, Y. Chen, C. Wang, S. Fan, Cryogenic 3D printing of bifunctional silicate nanoclay incorporated scaffolds for promoted angiogenesis and bone regeneration. Mater. Des. 223, 111220 (2022)
Q. Zhao, J. Wang, H. Cui, H. Chen, Y. Wang, X. Du, Programmed shape-morphing scaffolds enabling facile 3D endothelialization. Adv. Funct. Mater. 28(29), 1801027 (2018)
Y.B. Lee, O. Jeon, S.J. Lee, A. Ding, D. Wells, E. Alsberg, Induction of four-dimensional spatiotemporal geometric transformations in high cell density tissues via shape-changing hydrogels. Adv. Funct. Mater. 31(24), 2010104 (2021)
H. Naujokat, A.I. Gökkaya, Y. Açil, K. Loger, T. Klüter, S. Fuchs, J. Wiltfang, In vivo biocompatibility evaluation of 3D-printed nickel–titanium fabricated by selective laser melting. J. Mater. Sci. 33(2), 13 (2022)
M.A. Wagner, J.L. Ocana-Pujol, A. Hadian, F. Clemens, R. Spolenak, Filament extrusion-based additive manufacturing of NiTi shape memory alloys. Mater. Des. 225, 111418 (2023)
H. Hwangbo, H. Lee, E.J. Roh, W. Kim, H.P. Joshi, S.Y. Kwon, U.Y. Choi, I.-B. Han, G.H. Kim, Bone tissue engineering via application of a collagen/hydroxyapatite 4D-printed biomimetic scaffold for spinal fusion. Appl. Phys. Rev. 8(2), 021403 (2021)
Acknowledgments
M. Wang thanks Hong Kong’s Research Grants Council (RGC) for the financial support for his research on additive manufacturing and tissue engineering through various research grants, including recent Grants 17200519, 17202921, 17201622 and N_HKU749/22, and also The University of Hong Kong (HKU) through research grants in its Seed Fund for Basic Research Scheme. He thanks a donor in Hong Kong for her generous donation to support his research in biomaterials and tissue engineering. J. Lai thanks HKU for awarding him a PhD scholarship for conducting research at HKU. Assistance provided by members of M. Wang’s research group at HKU and by technical staff in HKU’s Department of Mechanical Engineering, Faculty of Medicine, Faculty of Dentistry and Electron Microscopy Unit is acknowledged.
Author information
Authors and Affiliations
Contributions
JL and MW: design of the review; JL: research and drafting of the manuscript; MW: concept development of 5D printing, research supervision, writing, reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
43578_2023_1193_MOESM1_ESM.docx
A shape memory cycle for 4D printed objects (Figure S1). Examples of 3D/4D printed tissue engineering scaffolds (Figures S2–S6). Summary of recent work on 3D/4D printing for different tissue engineering applications (Table S1–S7). Typical polymers for 3D/4D/5D printing in tissue engineering (Table S8). Supplementary file1 (DOCX 7479 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, J., Wang, M. Developments of additive manufacturing and 5D printing in tissue engineering. Journal of Materials Research 38, 4692–4725 (2023). https://doi.org/10.1557/s43578-023-01193-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-01193-5