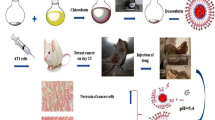
Abstract
In our previous paper, we demonstrated the ex vivo studies of non-toxic liposome-nanogel systems by which the long-term drug release could be provided from hybrid systems for the 5-fluorouracil (5-FU) drug molecule. The aim of this study was the in vivo magnetic targeting of 5-FU-loaded Fe3O4 nanoparticles including DPPC liposome-based PEGylated nanogels (5-FU loaded Fe3O4LPN) to breast cancer tissue and the investigation of the treatment and cytotoxic effects of that hybrid system to the liver and kidney in CD-1 mice using an external magnetic field. The effectiveness of the control, 5-FU group, Fe3O4LPN, and 5-FU-loaded Fe3O4LPN systems was evaluated using histopathology in terms of p53, ESR, PRG and C-erB-2, and qRT-PCR in terms of TYMS, ESR-1, RPG, and EGRF. Also, the cytotoxicity was analyzed by histopathological evaluation of kidney and liver tissues. Caspase-3 and caspase-9 evaluations were performed by qRT-PCR. The creatinine and ALT levels were also evaluated by comparing the blood samples of all groups. A total of 300-nm TEM-sized Fe3O4LNP hybrid system was successfully prepared. That system significantly decreased the TYMS and ESR1 levels after treatment process and increased the levels of p53 expression. The levels of caspase-3 mRNA did not change during the treatment, but the level of caspase-9 mRNA level was significantly decreased. The magnetically targeted liposome-based nanogel hybrid system is promising an effective therapy for the breast tumor with less liver and kidney damage. This Fe3O4LNP hybrid system could be useful for the similar small molecules.
Graphical Abstract










Similar content being viewed by others
References
Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. https://doi.org/10.3390/ijms21093233.
Kuo MT. Redox regulation of multidrug resistance in cancer chemotherapy: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2009;11(1):99–133. https://doi.org/10.1089/ars.2008.2095.
Nooter K, Stoter G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol Res Pract. 1996;192(7):768–80. https://doi.org/10.1016/S0344-0338(96)80099-9.
Jones AAD, Mi GJ, Webster TJ. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 2019;37(2):117–20. https://doi.org/10.1016/j.tibtech.2018.06.003.
Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3): e10143. https://doi.org/10.1002/btm2.10143.
Bobo D, Robinson KJ, Islam J, et al. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–87. https://doi.org/10.1007/s11095-016-1958-5.
Mu W, Chu Q, Liu Y, et al. A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nano-Micro Lett. 2020;12(142). https://doi.org/10.1007/s40820-020-00482-6.
Muhamad N, Plengsuriyakarn T, Na-Bangchang K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: a systematic review. Int J Nanomedicine. 2018;13:3921–35. https://doi.org/10.2147/IJN.S165210.
Sanna V, Sechi M. Therapeutic potential of targeted nanoparticles and perspective on nanotherapies. ACS Med Chem Lett. 2020;11(6):1069–73. https://doi.org/10.1021/acsmedchemlett.0c00075.
Li HF, Wu C, Xia M, et al. Targeted and controlled drug delivery using a temperature and ultra-violet responsive liposome with excellent breast cancer suppressing ability. RSC Adv. 2015;5:27630–9. https://doi.org/10.1039/C5RA01553G.
Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46. https://doi.org/10.1016/j.jconrel.2010.08.027.
Le Renard PE, Jordan O, Faes A, et al. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterial. 2010;31(4):691–705. https://doi.org/10.1016/j.biomaterials.2009.09.091.
Lu AH, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int. 2007;46(8):1222–44. https://doi.org/10.1002/anie.200602866.
Arias JL, Gallardo V, Ruiz A, et al. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-Fluorouracil delivery systems for active targeting. Eur J Pharm Biopharm. 2008;69(1):54–63. https://doi.org/10.1016/j.ejpb.2007.11.002.
Jafari S, Soleimani M, Salehi R. Nanotechnology-based combinational drug delivery systems for breast cancer treatment. Int J Poly Mater. 2019;68(14):859–69. https://doi.org/10.1080/00914037.2018.1517348.
Ahmed KS, Hussein SA, Ali AH, et al. Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J Drug Target. 2019;27(7):742–61. https://doi.org/10.1080/1061186X.2018.1527337.
Scherphof G, Roerdink F, Hoekstra D, Zborowski J, Gregoriadis EW, Allison AC. Liposomes in biological systems. New York: Wiley; 1980. p. 179–209.
Karami N, Moghimipour E, Salimi A. Liposomes as a novel drug delivery system: fundamental and pharmaceutical application. Asian J Phar. 2018;12(1):S31–41. https://doi.org/10.22377/AJP.V12I01.2037.
Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artificial Cells Nanom Biotech Int J. 2016;44(1):381–91. https://doi.org/10.3109/21691401.2014.953633.
Beltron-Gracia E, Lopez-Camacho A, Higuera-Ciapara I, et al. Nanomedicine review: clinical developments in liposomal applications. Cancer Nanotechnol. 2019;10(11). https://doi.org/10.1186/s12645-019-0055-y.
Smith CE, Kong H. Cross-linkable liposomes stabilize a magnetic resonance contrast-enhancing polymeric fastener. Langmuir. 2014;30(13):3697–704. https://doi.org/10.1021/la500412r.
Anilkumar TS, Lu YJ, Chen HA, et al. Dual targeted magnetic photosensitive liposomes for photothermal/photodynamic tumor therapy. J Magn Magn Mater. 2019;473:241–52. https://doi.org/10.1016/j.jmmm.2018.10.020.
Cern A, Nativ-Roht E, Goldblum A, et al. Effect of solubilizing agents on mupirocin loading into and release from PEGylated nanoliposomes. J Pharm Sci. 2014;103(7):2131–8. https://doi.org/10.1002/jps.24037.
Eloy JO, Petrilli R, Trevizan LNF, et al. Immunoliposomes: a review on functionalization strategies and targets for drug delivery. Colloid Surface B-Biointerfaces. 2017;159:454–67. https://doi.org/10.1016/j.colsurfb.2017.07.085.
Khan AA, Allemailem KS, Almatroodi SA, et al. Recent strategies towards the surface modification of liposomes: an innovative approach for different clinical applications. 3 Biotech. 2020;10(4):163. https://doi.org/10.1007/s13205-020-2144-3.
Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):381–91. https://doi.org/10.3109/21691401.2014.953633.
Kazakov S. Liposome-nanogel structures for future pharmaceutical applications. Curr Pharm Des. 2006;12(36):4713–28. https://doi.org/10.2174/1381612822666160125114733.
Grijalvo S, Mary J, Eritja R, et al. Biodegradable liposome-encapsulated hydrogels for biomedical applications: a marriage of convenience. Biomater Sci. 2016;4(4):555–74. https://doi.org/10.1039/C5BM00481K.
Torres-Martinez A, Cngulo-Pachon CA, Galindo F, et al. Liposome-enveloped molecular nanogels. Langmuir. 2019;35(41):13375–81. https://doi.org/10.1021/acs.langmuir.9b02282.
Allard-Vannier E, Cohen-Jonathan S, Gautier J, et al. PEGylated magnetic nanocarriers for doxorubicin delivery: a quantitative determination of stealthiness in vitro and in vivo. Eur J Pharm Biopharm. 2012;81(3):498–505. https://doi.org/10.1016/j.ejpb.2012.04.002.
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–8. https://doi.org/10.1038/nrc1074.
Zheng XF, Lian Q, Yang H, et al. Surface molecularly imprinted polymer of chitosan grafted poly(methyl methacrylate) for 5-fluorouracil and controlled release. Sci Rep. 2016;6:21409. https://doi.org/10.1038/srep21409.
Fournier E, Passirani C, Colin N, et al. Development of novel 5-FU-loaded poly(methylidene malonate 2.1.2)-based microspheres for the treatment of brain cancers. Eur J Pharm Biopharm. 2004;57(2):189–97. https://doi.org/10.1016/S0939-6411(03)00146-2.
Zhang W, Feng M, Zheng G, et al. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cell. Biochem Biophys Res Commun. 2012;417(2):679–85. https://doi.org/10.1016/j.bbrc.2011.11.142.
Zhang N, Yin Y, Xu SJ, et al. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13(8):1551–69. https://doi.org/10.3390/molecules13081551.
Hernandez-Vargas H, Ballestar E, Carmona-Saez P, et al. Transcriptional profiling of MCF7 breast cancer cells in response to 5-Fluorouracil: relationship with cell cycle changes and apoptosis, and identification of novel targets of p53. Int J Cancer. 2006;119(5):1164–75. https://doi.org/10.1002/ijc.21938.
Martignoni M, Kanter R, Moscone A, et al. Lack of strain-related differences in drug metabolism and efflux transporter characteristics between CD-1 and athymic nude mice. Cancer Chemother Pharmacol. 2005;55(2):129–35. https://doi.org/10.1007/s00280-004-0898-7.
Wang W, Nag S, Zhang RW. Pharmacokinetics and pharmacodynamics in breast cancer animal models. Breast Cancer: Methods and Protocols. 2016;1406:271–87. https://doi.org/10.1007/978-1-4939-3444-7_23.
Holliday DL, Speir V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13(4):215. https://doi.org/10.1186/bcr2889.
Horwitz KB, Costlow ME, McGuire WL. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975;26(6):785–95. https://doi.org/10.1016/0039-128X(75)90110-5.
Hashemi-Moghaddama H, KazemBagsangani S, Jamili M, et al. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int J Phar. 2016;497(1–2):228–38. https://doi.org/10.1016/j.ijpharm.2015.11.040.
Breast Cancer Hormone Receptor Status. The American Cancer Society medical and editorial content team. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancerdiagnosis/breast-cancer-hormone-receptor-status.html . Accessed 20 May 2021.
Pereira DM, Simoes AES, Gomes SE, et al. MEK5/ERK5 signaling inhibition increases colon cancer cell sensitivity to 5-fluorouracil through a p53-dependent mechanism. Oncotarget. 2016;7(23):34322–40. https://doi.org/10.18632/oncotarget.9107.
Kunnath AP, Kamaruzman NI, Chowdhury EH. Nanoparticle-facilitated intratumoral delivery of Bcl-2/IGF-1R siRNAs and p53 Gene synergistically inhibits tumor growth in immunocompetent mice. J Nanomed Nanotechnol. 2014;6:2. https://doi.org/10.4172/2157-7439.1000278.
Rashid S, Ali N, Nafees S, et al. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem Toxicol. 2014;66:185–93. https://doi.org/10.1016/j.fct.2014.01.026.
Aikemu A, Amat N, Yusup A, et al. Attenuation effect of Abnormal Savda Munziq on liver and heart toxicity caused by chemotherapy in mice. Exp Ther Med. 2016;12(1):384–90. https://doi.org/10.3892/etm.2016.3328.
Chenz M, He B, Wan T, et al. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE. 2012;7(10): e47115. https://doi.org/10.1371/journal.pone.0047115.
Yang T, Aimaiti M, Su D, et al. Enhanced efficacy with reduced toxicity of chemotherapy drug 5-fluorouracil by synergistic treatment with Abnormal Savda Munziq from Uyghur medicine. BMC Complement Altern Med. 2017;17(1):201. https://doi.org/10.1186/s12906-017-1685-4.
Keisuke K. Caspase-9. Int J Biochem Cell Biol. 2000;32(2):121. https://doi.org/10.1016/S1357-2725(99)00024-2.
Hsu TH, Hung SW, Wu CY, et al. Supplementation of beef extract improves chemotherapy-induced fatigue and toxic effects in mice. J Funct Foods. 2020;75. https://doi.org/10.1016/j.jff.2020.104232.
Ulker D, Barut I, Sener E, et al. Advanced liposome based PEGylated microgel as a novel release system for 5-fluorouracil against MCF-7 cancer cell. Eur Poly J. 2021;146:110270. https://doi.org/10.1016/j.eurpolymj.2021.110270.
Ulker D, Tuncer C, Sezgin SB, et al. An antibacterial composite system based on multi-responsive microgels hosting monodisperse gold nanoparticles. J Poly Res. 2017;24(10):1–11. https://doi.org/10.1007/s10965-017-1336-y.
Kirby C, Gregoriadis G. Dehydration-rehydration vesicles - a simple method for high-yield drug entrapment in liposomes. Bio-Technology. 1984;2(11):979–84. https://doi.org/10.1038/nbt1184-979.
Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108(6):2064–211. https://doi.org/10.1021/cr068445e.
Rahimi M, Yousef M, Cheng Y, et al. Formulation and characterization of a covalently coated magnetic nanogel. J Nanosci Nanotechnol. 2009;9(7):4128–34. https://doi.org/10.1166/jnn.2009.M21.
Kuhn DM, Balkıs M, Chandra J, et al. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. JCM. 2003;41(1):506–8. https://doi.org/10.1128/JCM.41.1.506-508.2003.
Lizuka K, Jin C, Eshima K, et al. Anti-cancer activity of the intraperitoneal-delivered DFP-10825, the cationic liposome-conjugated RNAi molecule targeting thymidylate synthase, on peritoneal disseminated ovarian cancer xenograft model. Drug Des Devel Ther. 2018;12:673–83. https://doi.org/10.2147/DDDT.S156635.
Sultana S, Alzahrani N, Alzahrani R, et al. Stability issues and approaches to stabilised nanoparticles based drug delivery system. J Drug Target. 2020;28(5):468–86. https://doi.org/10.1080/1061186X.2020.1722137.
Suk SJ, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(part A):28–51. https://doi.org/10.1016/j.addr.2015.09.012.
Marsh S. Thymidylate synthase pharmacogenetics. Invest New Drugs. 2005;23(6):533–7. https://doi.org/10.1007/s10637-005-4021-7.
Sasaki S, Watanabe T, Nakayama H. Analysis of the mRNA expression of chemotherapy-related genes in colorectal carcinoma using the Danenberg tumor profile method. J Oncol. 2013;2013: 386906. https://doi.org/10.1155/2013/386906.
Saga Y, Suzuki M, Mizukami H, et al. Overexpression of thymidylate synthase mediates desensitization for 5-fluorouracil of tumor cells. Int J Cancer. 2003;106(3):324–6. https://doi.org/10.1002/ijc.11221.
Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22(3):529–36. https://doi.org/10.1200/JCO.2004.05.064.
Kamoshida S, Suzuki M, Shimomura R, et al. Immunostaining of thymidylate synthase and p53 for predicting chemoresistance to S-1/cisplatin in gastric cancer. BJC. 2007;96(2):277–83. https://doi.org/10.1038/sj.bjc.6603546.
Garbar C, Mascaux C, Giustiniani J, et al. Chemotherapy treatment induces an increase of autophagy in the luminal breast cancer cell MCF7, but not in the triple-negative MDA-MB231. Sci Rep. 2017;7(1):7201. https://doi.org/10.1038/s41598-017-07489-x.
Acknowledgements
We are grateful to Prof. Dr. Emine Dündar of ESOGU of the Medical Pathology for helping us in the pathological and immunohistochemical evaluation. We are also thankful to the veterinarian Salih Salar of KOBAY DLH A.Ş. Experimental Animal Centre for providing the breast cancer model.
Funding
We thank for the financial support of both the Scientific and Technological Research Council of Turkey (grant number: 115Z726 and 1649B03140610 - 2211/C) and the Scientific Research Projects Commission of ESOGU (grand number: 201719D05, 201619C104).
Author information
Authors and Affiliations
Contributions
Damla Ulker: visualization, methodology, conceptualization, writing—review and editing, validation, writing – original draft, data curation. Rumeysa Ozyurt: validation, data curation, qRT-PCR experiments. Nilufer Erkasap and Vural Butun: supervision, funding acquisition, resources, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ulker, D., Ozyurt, R., Erkasap, N. et al. Magnetic Targeting of 5-Fluorouracil-Loaded Liposome-Nanogels for In Vivo Breast Cancer Therapy and the Cytotoxic Effects on Liver and Kidney. AAPS PharmSciTech 23, 289 (2022). https://doi.org/10.1208/s12249-022-02438-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02438-y