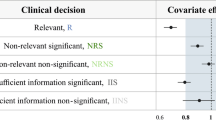
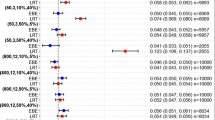
Abstract
One important objective of population pharmacokinetic (PPK) analyses is to identify and quantify relationships between covariates and model parameters such as clearance and volume. To improve upon existing covariate model development methods including stepwise procedures and Wald’s approximation method (WAM), this paper introduces an innovative method named the hybrid first-order conditional estimation (FOCE)/Monte-Carlo parametric expectation maximization (MCPEM)-based Wald’s approximation method with backward elimination (BE), or H-WAM-BE. Compared with WAM, this new method uses MCPEM to obtain full covariance matrix after running FOCE to obtain full model parameter estimates, followed by BE to select the final covariate model. Two groups of datasets (simulation datasets and rituximab datasets) were used to compare the performance of H-WAM-BE with two other methods, likelihood ratio test (LRT)-based stepwise covariate method (SCM) and H-WAM with full subset approach (H-WAM-F) in NONMEM. Different scenarios with different sample sizes and sampling schemes were used for simulating datasets. The nominal model was used as the reference to evaluate the three methods for their ability to accurately identify parameter-covariate relationships. The methods were compared using the number of true and false positive covariates identified, number of times that they identified the reference model, computation times, and predictive performance. Best-performing H-WAM-BE methods (M2 and M4) showed comparable results with LRT-based SCM. H-WAM-BE required shorter or comparable computation times than LRT-based SCM and H-WAM-F regardless of the model structure, sample size, or sampling design used in this study.




Similar content being viewed by others
References
Hutmacher MM, Kowalski KG. Covariate selection in pharmacometric analyses: a review of methods. Br J Clin Pharmacol. 2015;79(1):132–47.
Khandelwal A, Harling K, Jonsson EN, Hooker AC, Karlsson MO. A fast method for testing covariates in population PK/PD Models. AAPS J. 2011;13(3):464.
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokineticpharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20(5):511–28.
Ribbing J, Nyberg J, Caster O, Jonsson EN. The lasso—a novel method for predictive covariate model building in nonlinear mixed effects models. J Pharmacokinet Pharmacodyn. 2007;34(4):485–517.
Jonsson EN, Karlsson MO. Automated covariate model building within NONMEM. Pharm Res. 1998;15(9):1463–8.
Kowalski KG, Hutmacher MM. Efficient screening of covariates in population models using Wald's approximation to the likelihood ratio test. J Pharmacokinet Pharmacodyn. 2001;28(3):253–75.
Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–4.
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM users guides. NONMEM Project Group. San Francisco: University of California; 1992.
Bauer RJ, Guzy S, Ng C. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J. 2007;9(1):E60–83.
Ng CM, Patnaik A, Beeram M, Lin C, Takimoto C. Mechanism-based pharmacokinetic/pharmacodynamic model for troxacitabine-induced neutropenia in cancer patients. Cancer Chemother Pharmacol. 2011;67(5):985–94.
Ng CM, Joshi A, Dedrick RL, Garovoy MR, Bauer RJ. Pharmacokinetic–pharmacodynamic–efficacy analysis of efalizumab in patients with moderate to severe psoriasis. Pharm Res. 2005;22(7):1088–100.
Walker S. An EM algorithm for nonlinear random effects models. Biometrics. 1996:934–44.
Schumitzky A. EM algorithms and two stage methods in pharmacokinetic population analysis. Adv Methods Pharmacokinet Pharmacodyn Syst Anal. 1995;2:145–60.
National Health and Nutrition Examination Survey, 2017 - 2018 Data Documentation, Codebook, SAS Code. Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx? BeginYear=2017. Accessed 20 Oct 2020.
Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45(7):792–801.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition (Burbank, Los Angeles County, Calif). 1989;5(5):303–11 discussion 12-3.
Lehmann EL, Romano JP. Testing statistical hypotheses: Springer Science & Business Media; 2006.
Trevethan R. Sensitivity, specificity, and predictive values: foundations, pliabilities, and pitfalls in research and practice. Front Public Health. 2017;5:307.
Rao C. Score test: historical review and recent developments. Advances in Ranking and Selection, Multiple Comparisons, and Reliability. Springer; 2005. p. 3–20.
Buse A. The likelihood ratio, Wald, and Lagrange multiplier tests: An expository note. Am Stat. 1982;36(3a):153–7.
Basile AS, Hutmacher MM, Kowalski KG, Gandelman KY, Nickens DJ. Population pharmacokinetics of pegaptanib sodium (Macugen((R))) in patients with diabetic macular edema. Clin Ophthalmol (Auckland NZ). 2015;9:323–35.
Cirincione B, Kowalski K, Nielsen J, Roy A, Thanneer N, Byon W, et al. Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst Pharmacol. 2018;7(11):728–38.
Luo M, Chapel S, Sevinsky H, Savant I, Cirincione B, Bertz R, et al. Population pharmacokinetics analysis to inform efavirenz dosing recommendations in pediatric HIV patients aged 3 months to 3 years. Antimicrob Agents Chemother. 2016;60(6):3676–86.
Wilks SS. The large-sample distribution of the likelihood ratio for testing composite hypotheses. Ann Math Stat. 1938;9(1):60–2.
Bonate PL, Steimer J-L. Pharmacokinetic-pharmacodynamic modeling and simulation: Springer; 2011.
Byon W, Smith M, Chan P, Tortorici M, Riley S, Dai H, et al. Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst Pharmacol. 2013;2(7):1–8.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
ALB, Albumin; ALK, Alkaline phosphatase; ALT, Alanine transaminase; AST, Aspartate transaminase; BE, Backward elimination; BSA, Body surface area; CL, Clearance; COV, Variance-covariance matrix; EM, Expectation maximization; FOCE, First-order conditional estimation; FS, Forward selection; GEN, Gender; H-WAM-BE, Hybrid first-order conditional estimation/Monte-Carlo parametric expectation maximization-based Wald’s approximation method with backward elimination; H-WAM-F, H-WAM with full subset approach; HCT, Hematocrit; IV, Intravenous; LDH, Lactate dehydrogenase; LRT, Likelihood ratio test; M0a, H-WAM-BE interim model with pbe1 = 0.05; M0b, H-WAM-BE interim model with pbe1 = 0.01; M1, H-WAM-BE pbe1 = 0.05 & pbe2 = 0.05; M2, H-WAM-BE pbe1 = 0.05 & pbe2 = 0.01; M3, H-WAM-BE pbe1 = 0.01 & pbe2 = 0.05; M4, H-WAM-BE pbe1 = 0.01 & pbe2 = 0.01; MCPEM, Monte-Carlo parametric expectation maximization; NHANES, National Health and Nutrition Examination Survey; OFV, Objective function value; PC, Platelet count; PD, Pharmacodynamic; PK, Pharmacokinetic; PPK, Population pharmacokinetics; Q, Distribution clearance; RMSE, Root mean squared error; SBC, Schwarz’s Bayesian criterion; SCR, Serum creatinine; SCM, Stepwise covariate method; SCM1, SCM with pfs = 0.05 and pbe = 0.01; SCM2, SCM with pfs = 0.01 and pbe = 0.01; TB, Total bilirubin; V, Volume of distribution; Vc, Volume of the central compartment; Vp, Volume of the peripheral compartment; WAM, Wald’s approximation method; WT, Weight
Supplementary Information
ESM 1
(DOCX 311 kb)
Rights and permissions
About this article
Cite this article
Zou, Y., Tang, F. & Ng, C.M. A Modified Hybrid Wald’s Approximation Method for Efficient Covariate Selection in Population Pharmacokinetic Analysis. AAPS J 23, 37 (2021). https://doi.org/10.1208/s12248-021-00572-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00572-2