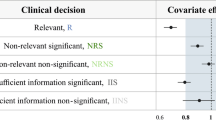
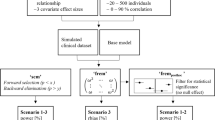
Abstract
Covariate analysis based on population pharmacokinetics (PPK) is used to identify clinically relevant factors. The likelihood ratio test (LRT) based on nonlinear mixed effect model fits is currently recommended for covariate identification, whereas individual empirical Bayesian estimates (EBEs) are considered unreliable due to the presence of shrinkage. The objectives of this research were to investigate the type I error for LRT and EBE approaches, to confirm the similarity of power between the LRT and EBE approaches from a previous report and to explore the influence of shrinkage on LRT and EBE inferences. Using an oral one-compartment PK model with a single covariate impacting on clearance, we conducted a wide range of simulations according to a two-way factorial design. The results revealed that the EBE-based regression not only provided almost identical power for detecting a covariate effect, but also controlled the false positive rate better than the LRT approach. Shrinkage of EBEs is likely not the root cause for decrease in power or inflated false positive rate although the size of the covariate effect tends to be underestimated at high shrinkage. In summary, contrary to the current recommendations, EBEs may be a better choice for statistical tests in PPK covariate analysis compared to LRT. We proposed a three-step covariate modeling approach for population PK analysis to utilize the advantages of EBEs while overcoming their shortcomings, which allows not only markedly reducing the run time for population PK analysis, but also providing more accurate covariate tests.






Similar content being viewed by others
References
Duan JZ. Applications of population pharmacokinetics in current drug labelling. J Clin Pharm Ther. 2007;32(1):57–79.
Menon-Andersen D, Yu B, Madabushi R, Bhattaram V, Hao W, Uppoor RS, et al. Essential pharmacokinetic information for drug dosage decisions: a concise visual presentation in the drug label. Clin Pharmacol Ther. 2011;90(3):471–4.
Joerger M. Covariate pharmacokinetic model building in oncology and its potential clinical relevance. AAPS J. 2012;14(1):119–32. Pubmed Central PMCID: 3291194.
Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28(3):231–52.
Maitre PO, Buhrer M, Thomson D, Stanski DR. A three-step approach combining Bayesian regression and NONMEM population analysis: application to midazolam. J Pharmacokinet Biopharm. 1991;19(4):377–84.
Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic—pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20(5):511–28.
Lindbom L, Ribbing J, Jonsson EN. Perls-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Meth Prog Biomed. 2004;75(2):85–94 PubMed PMID: WOS:000222690900001. English.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69. PubMed PMID: WOS:000270544500018. English.
Xu XS, Yuan M, Karlsson MO, Dunne A, Nandy P, Vermeulen A. Shrinkage in nonlinear mixed-effects population models: quantification, influencing factors, and impact. AAPS J. 2012;14(4):927–36. PubMed PMID: WOS:000310367100030. English.
Karlsson MO, Savic RM. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82(1):17–20.
Combes FP, Retout S, Frey N, Mentre F. Powers of the likelihood ratio test and the correlation test using empirical bayes estimates for various shrinkages in population pharmacokinetics. CPT Pharmacometrics Syst Pharmacol. 2014;3:e109. Pubmed Central PMCID: 4011164.
Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer; 2000. xvi, 528 p.
R Development Core Team. R: A Language and Environment for Statistical Computing (www.r-project.org). Vienna, Austria: R Foundation for Statistical Computing; 2008.
Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Effect. 2012;5(2):189–211.
Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8(6):553–71.
Vonesh EF, Carter RL. Mixed-effects nonlinear regression for unbalanced repeated measures. Biometrics. 1992;48(1):1–17.
Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46(3):673–87.
Davidian M, Gallant AR. Smooth nonparametric maximum likelihood estimation for population pharmacokinetics, with application to quinidine. J Pharmacokinet Biopharm. 1992;20(5):529–56.
Samson A, Lavielle M, Mentre F. The SAEM algorithm for group comparison tests in longitudinal data analysis based on non-linear mixed-effects model. Stat Med. 2007;26(27):4860–75.
Davidian M, Giltinan DM. Nonlinear models for repeated measurement data: an overview and update. J Agric Biol Environ Stat. 2003;8(4):387–419.
Acknowledgments
There is no conflict of interest. Dr. Min Yuan is supported by the National Science Foundation of China (NSFC), Grant No. 11271346 and the Fundamental Research Funds for the Central Universities (No.WK0010000052).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xu Steven Xu and Min Yuan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 55 kb)
ESM 2
(DOC 50 kb)
ESM 3
(DOC 765 kb)
ESM 4
(DOC 61 kb)
Supplementary Fig. 1
Comparison of root mean square error between likelihood ratio test (LRT) and EBEs. The size of each symbol is scaled based on the number of successful runs for the corresponding simulation scenario. (GIF 19 kb)
Rights and permissions
About this article
Cite this article
Xu, X.S., Yuan, M., Yang, H. et al. Further Evaluation of Covariate Analysis using Empirical Bayes Estimates in Population Pharmacokinetics: the Perception of Shrinkage and Likelihood Ratio Test. AAPS J 19, 264–273 (2017). https://doi.org/10.1208/s12248-016-0001-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-0001-4