Abstract
In vivo analyses of pharmacological data are traditionally based on a closed system approach not incorporating turnover of target and ligand-target kinetics, but mainly focussing on ligand-target binding properties. This study incorporates information about target and ligand-target kinetics parallel to binding. In a previous paper, steady-state relationships between target- and ligand-target complex versus ligand exposure were derived and a new expression of in vivo potency was derived for a circulating target. This communication is extending the equilibrium relationships and in vivo potency expression for (i) two separate targets competing for one ligand, (ii) two different ligands competing for a single target and (iii) a single ligand-target interaction located in tissue. The derived expressions of the in vivo potencies will be useful both in drug-related discovery projects and mechanistic studies. The equilibrium states of two targets and one ligand may have implications in safety assessment, whilst the equilibrium states of two competing ligands for one target may cast light on when pharmacodynamic drug-drug interactions are important. The proposed equilibrium expressions for a peripherally located target may also be useful for small molecule interactions with extravascularly located targets. Including target turnover, ligand-target complex kinetics and binding properties in expressions of potency and efficacy will improve our understanding of within and between-individual (and across species) variability. The new expressions of potencies highlight the fact that the level of drug-induced target suppression is very much governed by target turnover properties rather than by the target expression level as such.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Background
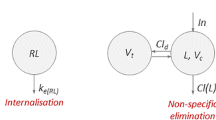
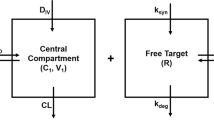
In this paper, we continue our study of in vivo potency of drug-target kinetics begun in Gabrielsson, Peletier et al. and Hjorth et al. (1,2) in the framework of Target-Mediated Drug Disposition (TMDD), an ubiquitous process in the action of drugs that has been extensively studied ever since the pioneering papers of Wagner (3), Sugiyama et al. (4) and Levy (5). We also refer to the seminal papers by Michaelis and Menten (6), Mager and Jusko (7), Mager and Krzyzansky (8), Gibiansky et al. (9) and Peletier and Gabrielsson (10). In Fig. 1, we show schematically the basic TMDD model: Ligand is supplied to the central compartment where it binds a receptor (the target) resulting in a ligand-receptor complex, which internalises to produce a pharmacologial response. In addition, ligand is cleared from the central compartment and exchanged with a peripheral compartment. Target is synthesised by a zeroth order process and degrades by a first-order process.
In this paper, we extend the results for this TMDD model obtained in (1) to three generalisations of the TMDD model in which (i) the drug can bind two receptors (cf. 11), (ii) two drugs can bind one receptor (cf. 12) and (iii) the dug is supplied to the central compartment, but the receptor is located in the peripheral compartment (cf. 13).
Mathematically, the basic TMDD model, depicted in Fig. 1, can be formulated as a set of four differential equations, one for each compartment.
Here, Lc and Lp denote the concentrations of ligand (or drug) in, respectively, the central and the peripheral compartment with volumes Vc and Vp. Concentrations of target and target-ligand complex in the central compartment are denoted by R and RL. Drug infusion takes place into the central compartment, with constant rate In where it binds to the target with rates kon and koff. Ligand is removed through non-specific clearance Cl(L) and exchanged with the peripheral compartment through inter-compartmental distribution Cld. By internalisation, ligand-target complex leaves the system according to a first-order process with a rate constant ke(RL). Finally, target synthesis and degradation are modelled by, respectively, zeroth- and first-order turnover with rates ksyn and kdeg.
We recall the analysis presented in (1) for the one-compartment TMDD-model shown in Fig. 1. There, relations between steady-state concentrations of target R, ligand L and complex RL were derived, and a new expression of the in vivo potency, denoted by L50, was established, particularly suited for Open Systems. Whereas the classical definition of potency is primarily based on the binding constants (cf. Black and Leff (14), Kenakin (15,16), Neubig et al. (17)) and target expression, in the definition of, in vivo potency drug and target kinetics, such as the degradation rate kdeg, are also incorporated. These concepts were further discussed from an open and closed system perspective in (2).
In this paper, we present three generalisations of the classical TMDD model: (i) a single ligand that can bind two receptors R1 and R2, (ii) two ligands, L1 and L2, that compete for a single receptor and (iii) a ligand that is supplied to the central compartment and distributed to the peripheral compartment where the target is located.
Steady States
In (1), it has been established how for the model shown in Fig. 1, the steady-state values of ligand (L), receptor (R) and ligand-receptor complex (RL), in the central compartment, are related to one another:
where the baseline R0, the maximal impact R∗ and the in vivo potency EC50 (denoted by L50) are given by
and Km = (koff + ke(RL))/kon is called the Michaelis-Menten constant. Here, it is implicitly assumed that the constant rate infusion, In, is fixed at the appropriate value. In (1), the required infusion rate is also computed.
The definition of the in vivo potency, L50, expresses both the impact of rate processes of the target (kdeg, ke(RL)) and those of the binding dynamics (koff, kon), on the drug concentration (L) required to achieve the desired efficacy.
The resemblance of Eq. (2) with the Hill equation (below) is striking.
The Hill equation is often used in vivo and also contains a baseline parameter E0 in addition to the maximum drug induced effect Emax and the potency EC50. Equation (2) has intrinsically the baseline in terms of R0. The Emax parameter is equivalent to ∣RLmax − R0∣, and the potency parameter EC50 is expressed in Eq. (3) as L50.
The exponent nH of the Hill equation is interpreted as a fudge factor allowing the steepness of the Hill equation at the EC50 value to vary. In our experience nH is not necessarily an integer and varies typically within the range of 1–3. We have observed with high and variable plasma protein binding that nH will change depending on whether unbound or total plasma concentration (respectively Cu and Ctot) is used as drivers of the pharmacological effect.
Remark
It is interesting to note that Eq. (3) yields the following relation between the baseline target concentration, R0, the maximal ligand-target concentration, R∗, and the in vivo potency L50:
This means that if the baseline of target, the maximum ligand-target concentration and Km are obtained experimentally, then the in vivo potency L50 can be predicted. Thus, Km can be located either to the right or to the left of the in vivo potency, depending on the relative magnitude of R0 and R∗.
In Fig. 2, we show graphs for RL and R versus L for two parameter sets, one taken from Peletier and Gabrielsson (10) (left) and one from Cao and Jusko (18) (right) (cf. Appendix 2; Tables I and II).
The values of R0, R∗ and L50 that appear in Eq. (2) are for these two references given by
Thus, remembering that initially, R = R0 and RL = 0, it is evident that over time, the system settles into a steady state, in (10) where total target concentration exceeds R0 and in (18) where target concentration is less than target baseline.
It is interesting to note that despite similar in vivo potency’s (L50’s) of Cao and Jusko and Peletier and Gabrielsson, the target-to-complex ratios differ by one order of magnitude due to the comparable difference in ke(RL).
The proposed framework with a dynamic target protein may also be applicable to enzymatic reactions which may enhance the in vitro/in vivo extrapolation of metabolic data (cf Pang et al. 19,20).
Discussion and Conclusions
Eqs. (2) and (3) summarise what is needed to apply and explain target R, ligand-target RL and ligand L interactions when both ligand and target belongs to the central (plasma) compartment. Equation (3) clearly demonstrates that in vivo potency, a central parameter in pharmacology, is a conglomerate of target turnover, complex kinetics and ligand-target binding properties.
In the following three sections, we discuss generalisations of the basic TMDD model discussed in “INTRODUCTION” and derive generalisations of the functions RL = f(L) and R = g(L) applicable to these models.
TWO DIFFERENT RECEPTORS COMPETE FOR ONE LIGAND
Background
When one ligand, L, can bind two receptors, R1 and R2, two complexes are formed and internalised to form two different ligand-receptor complexes R1L and R2L; it is of great value to determine their relative impact on the pharmacological response, and it is important to determine how the responses of these two complexes are related. For instance, when one receptor mediates a beneficial effect of a drug and the other one mediates an adverse effect, one wishes to know the relative impact of the latter target and whether the two potencies EC50; 1 and EC50; 2 are sufficiently well separated so that a dose can be selected with minimally adverse effect. Figure 3 gives a schematic description of the model.
Schematic description of a model for the one-compartment two-target system in which ligand binds with two receptors R1 and R2, each forming a complex denoted by, respectively, R1L and R2L. The definition of parameters is the same as in Fig. 1
Mathematically, the model shown in Fig. 3 can be described by the following system of ordinary differential equations:
in which the parameters are defined as in the system (1) and
For a related model, with a corresponding system of equations, we refer to (11). By adding the equations for free receptors R1 and R2 to the equations for the associated bound receptors R1L and R2L, we obtain two balance equations for, respectively, R1 and R2:
For ligand, free or bound to one of the two receptors, we obtain the balance equation:
Steady States
As in the case of a single target, it is possible to obtain expressions for the concentrations of ligand-target complex and free target, i.e. for RiL and Ri (i = 1, 2) in terms of the ligand concentration L.
Following the steps taken in Gabrielsson and Peletier (1), it is possible to show that for the receptors individually, the expressions such as shown in (2) hold
for i = 1 and i = 2.
The baseline receptor concentrations R0. i, the maximum values \( {R}_i^{\ast } \) of the receptor-ligand complexes and the in vivo potencies L50; i are given by
for i = 1 and i = 2.
where \( {K}_{m;i}=\left({k}_{\mathrm{off};i}+{k}_{e\left({R}_iL\right)}\right)/{k}_{\mathrm{on};i} \). Details of the derivations of the formulas above are presented in Appendix 1.1.
Figure 4 shows the target suppression and complex formation of the two targets versus ligand concentration at equilibrium together with their respective L50 values. These graphs are useful in discriminating between two targets and deciding which target contributes most to complex formation at different ligand concentrations. The left figure shows the two target suppression curves R1 and R2versus ligand and the right figure the two ligand-target complexes R1L and R2L versus ligand L. The parameter values are chosen fictitiously in order to clearly highlight the differences (Table III, Appendix 2).
The model shown in Fig. 3 has been used as a two-state model to fit the data for the total free target concentration that were given in Gabrielsson and Weiner (PD2) p. 729 (21). The total free target concentration, i.e. R1 + R2, can be computed from Eq. (10) and (11) and is seen to be
Evidently, in the absence of ligand, Rfree; tot = R0.1 + R0.2, while Rfree; tot → 0 as L → ∞.
In Fig. 5, we see how the model is fitted to data obtained from an experiment involving four total target concentrations (Rtot = 8050, 6510, 3540 and 1590 nM). As the ligand concentration increases, the first receptor kicks in at the lowest in vivo potency (0.025 nM), taking the free receptor concentration down to a lower intermediate plateau. Then, at the higher in vivo potency (37 nM), the free receptor concentration drops further and eventually converges to zero.
Total free target level Rtot;free = R1 + R2 and model predicted graphs (solid lines) of Rtot;freeversus L for four total receptor concentrations (Rtot = 8050, 6510, 3540 and 1590 nM) and R0,1 = Rtot · F and R0,2 = Rtot · (1 − F) with F = 0.6 Note the wide discrepancy between the two in vivo potencies L50;1 (denoted in the figure by IC50;1)(0.025 nM) and L50;2 (denoted in the figure by IC50;2) (37 nM). The high affinity drug is the target for therapeutic effect, and the low affinity drug is responsible for an adverse effect (cf. Gabrielsson and Weiner, PD2, page 729 21)
Remark. The parameters kdeg, ke(RL), kon and koff are not given here since only equilibrium data from the experiments were available. Due to parameter unidentifiability, the model was parametrised with potencies L50; 1 and L50; 2 as parameters and not functions of their original determinants. One may also need other sources of information to fully appreciate the actual values of kdeg, ke(RL), kon and koff. In vitro binding experiments may yield kon and koff. In vivo time courses of circulating free ligand, target and ligand-target are necessary in order to estimate ke(RL). Information about the kdeg parameter may be found in the literature for commonly studied targets.
Discussion and Conclusion
Here, Eq. (10), (11) and (12) summarise what is needed to apply and explain target Ri, ligand-target RiL and ligand L interactions when ligand and both targets belong to the central (plasma) compartment. Equation (11) demonstrates again the complexity of in vivo potencies L50; i involving both turnover of the two targets, complex kinetics and ligand-target binding properties.
The explicit expressions for the ligand-receptor complexes RiL, the free receptor concentrations Ri and the in vivo potencies L50; i (cf. (12)), together with Figs. 4 and 5, provide valuable tools when assessing the individual contribution of each target and specifically the impact of target turnover and internalisation.
TWO DIFFERENT LIGANDS COMPETING FOR ONE RECEPTOR
Background
A common situation, for instance in combination therapy, is that not one but two ligands L1 and L2 bind a single receptor R. This results in two different complexes, RL1 and RL2, with different internalisation rates. For instance, one of the ligands is produced endogenously, and the other is a drug which is supplied in order to inhibit or stimulate the pharmacological effect caused by the endogenous ligand (cf. Benson et al. 22,23).
Recently, several authors have derived different drug-drug interaction models associated with TMDD with a different focus and often directed towards the situation when a constant target level prevails (cf. Koch et al. (24,25) and Gibiansky et al. (26)). In order to describe open, in vivo, processes, it is necessary to include target turnover, internalisation and drug clearance. This is done in the model shown in Fig. 6 in which two ligands, distinguished by subscripts i = 1 and 2, are supplied by constant-rate infusions Ini to the central compartment, each having its own volume of distribution Vci, nonspecific clearance \( C{l}_{\left({L}_i\right)} \), binding and dissociation rate kon; i and koff; i, and its own internalisation rate \( {k}_{e\left(R{L}_i\right)} \).
Mathematically, the model shown in Fig. 6 can be described by the following system of differential equations for the two ligands, L1 and L2, the target R and the two ligand-target complexes RL1 and RL2 (see also (12)):
where
Each of the ligands is present in free form (Li) and in bound form (RLi) (i = 1, 2). For the total amount of the two ligands, we then find two balance equations, one for L1 and one for L2:
The receptor is present in free form (R) and in bound form (RLi). Adding the last three equations of the system (Eq. (13)), we obtain the following balance equation for the receptor:
These balance equations will be useful for analysing steady-state concentrations, when the left-hand sides vanish and we obtain three algebraic equations.
Steady States
We deduce from Eq. (16) that the steady-state concentrations R, RL1 and RL2 are related by the equation
This allows us to express R in terms of the concentrations of the two complexes, RL1 and RL2:
or
when we use the short-hand notation
We substitute the expression for R in Eq. (19) into the right-hand side of each of the last two equations of Eq. (13) to obtain:
where
This is an algebraic system of two equations with two unknowns, X1 and X2, which can be solved. Translating these solutions back to the original variables, we obtain the following expressions for RL1 and RL2:
where L50; 1 and L50; 2 are given by
for i = 1 and i = 2.
The expressions for the complexes RL1 and RL2 can be used in Eq. (18) to derive an expression for R in terms of the two ligand concentrations:
where θ = L50; 1/L50; 2. Thus, the impact of the two ligand combined is seen to be additive.
Details of the derivations of the equations above are given in Appendix 1.2.
In the expressions for RL1 in Eq. (22), one can interpret the term (θ · L2 + L50; 1) in the numerator as a shift of potency L50; 1, and similarly in the expression for RL2, the term (θ−1 · L1 + L50; 2) can be viewed as a shift of potency L50; 2. Thus, the modifications of the potencies L50; 1 and L50; 2 (equivalent to EC50; 1 and EC50; 2) depend on the ligand concentrations in the following manner:
i.e. EC50; 1increases when L2increases. Similarly, EC50;2 increases when L1 increases.
Note that by Eq. (22), when L2 is arbitrary but fixed, then
In the context of an endogenous ligand (L1) and a drug (L2) which is administered to reduce the effect of the endogenous ligand, Eq. (22) is of practical value. Assuming that receptor occupancy RL1 is a measure for the effect of L1, it tells by how much the effect of L1 is reduced by a given concentration of L2.
Finally, we observe that
These limits are consistent with the expression in Eq. (2) for a single receptor shown in “INTRODUCTION”. Plainly, RL1(L1, L2) = 0 when L1 = 0 and RL2(L1, L2) = 0 when L2 = 0.
It is illustrative to view the two complexes and the total free drug concentration as they depend on both ligand concentrations: L1 and L2. This is done in Fig. 7 where 3D graph of R versus L1 and L2 is shown as well as the corresponding Heat map. In both graphs, R0 = 100, L50; 1 = 50 and L50; 2 = 25 are taken so that θ = 2.
Graphs of R versus L1 and L2 according to Eq. (24). Here, R0 = 100, L50;1 = 50 and L50;2 = 25 so that θ = 2. Note that the level curves are straight lines with slope L1/L2 = − 2
As we see
These limits are in agreement with the Eq. (22) for RL1 and Eq. (25) for R.
Discussion and Conclusion
Equations (22), (23) and (24) summarise what is needed to apply and explain target R, ligand-target complex RLi and ligand Li interactions when two ligands interact with one centrally located target. Equation (23) clearly demonstrates that in vivo potency is a conglomerate of target turnover, complex kinetics and ligand-target binding properties.
TARGET IN THE PERIPHERAL COMPARTMENT
Background
When ligand and target are located in the central compartment of the TMDD model, the steady-state relations of ligand, target and ligand-target complex have been derived in Gabrielsson and Peletier (1) and briefly summarised in the “Introduction” (cf Eqs. (2) and (3)). In this section, we generalise this situation to when ligand is supplied to the central compartment, but target is located in the peripheral compartment so that ligand has to be cleared from the central compartment into the peripheral compartment before it can bind the target.
We assume active transport between the two compartments, as may be caused by blood flow or transporters, and denote clearance from the central compartment by Cldα and from the peripheral compartment by Cldβ. These two processes allow concentration differences to build up across the membrane separating the two compartments..
The objective is here to derive expressions for the concentration of free receptor R and ligand-receptor complex RLp in the peripheral compartment and the ligand concentration Lc in the central compartment.
Figure 8 gives a schematic description of the model we study.
The system (1) now becomes
For convenience, we shall often write
The system (28) yields the following balance equations for the target and the ligand:
-
For the target, which involves free target R and bound target RLp: By adding the third and fourth equation of Eq. (28), we obtain
-
For the ligand, which involves Lc, Lp and RLp: By adding the first equation in Eq. (28) and the sum of the second and the fourth equation multiplied by μ = Vp/Vc, we obtain
Steady States
An expression for the concentration of ligand-target complex in terms of the ligand concentration in the peripheral compartment Lp can be derived in a manner which is analogous to the one employed before for the one-compartment model and yields the following equation:
Note that the expression for Lp; 50 is the same as the one defined for L50 in Eq. (3).
Next, we replace Lp in the expression for RLp by Lc. By adding the second and the fourth equation of the system (Eq. (28)), we express Lp in terms of Lc and RLp:
When we use this equation in Eq. (32) to replace Lp by Lc, we arrive at an expression which only involves Lc and RLp. Specifically, putting \( X={k}_{e\left(R{L}_p\right)}R{L}_p \), we obtain
Equation (34) provides an expression for Lc as a function of X, i.e. Lc = f(X). It is seen that the function f(X) is monotonically increasing so that it can be inverted to give an expression of X in terms of Lc and so yield the desired expression of RLp in terms of Lc.
In order to invert the function f(X), we multiply Eq. (34) by (ksyn − X) and so obtain a quadratic equation in X:
in which
The roots of this equation are
Obviously, we need the root which vanishes when Lc = 0, i.e. we need X−. Therefore
The corresponding expression for target depression in terms of the ligand concentration in plasma (Lc) is found to be given by
A more detailed derivation of these expressions for the concentration of ligand-receptor complex in terms of the ligand concentration in plasma can be found in the Appendix 1.1 (A.3 and A.4).
On the basis of the implicit expression (34) of X (i.e. RLp) in terms of Lc, it is also possible to define Lc; 50. Plainly, at Lc; 50, we have X = ksyn/2, i.e. RLp = R∗/2. When we substitute this value for X into Eq. (34), we obtain the following formula for Lc; 50:
or, when we replace the rates kcp and kpc by clearances again, we obtain
where we have used the definition of Lp; 50 in Eq. (32).
Passive Transport Between Central and Peripheral Compartment
If distribution between the two compartments is passive, i.e. Cldα = Cldβ = Cld, then the expression for Lc; 50 reduces to
Observation
Equation (40) immediately implies that
If target-synthesis is small compared to in- and out-flow of ligand between the two compartments, the reverse inequalities are seen to hold as well.
It follows from this expression that Lc, 50increases when transport from the central towards the peripheral compartment becomes harder (Cldα↗) and vice versa, it decreases when it becomes easier (Cldβ↘).
If ksyn is small, more specifically, if
then, the expression (Eq. (40)) reduced to a particularly simple relation between Lc; 50 and Lp; 50:
in which the relative impact of the two clearances becomes very transparent.
The expression (Eq. (37)) for RLp in terms of Lc is fairly complex and not so easy to grasp. However, it is possible to derive a few properties of the dependence of RLp on Lc without going to the details of an explicit computation based on Eq. (37). Below we give a few examples.
-
1.
It follows from Eq. (37) that 0 < X < ksyn. Therefore, remembering that \( X={k}_{e\left(R{L}_p\right)}R{L}_p \), it follows that
regardless of the ligand concentration Lc in the central compartment.
-
2.
It is clear from Eq. (34) that Lc is an increasing function of X. Therefore, RLp is an increasing function of Lc:
Note that in Eqs. (44) and (45), the active transport between the central and the peripheral compartment (the parameters α and β) do not come into the upper bound and the limit for large Lc.
In Fig. 9, we show graphs of R and RLpversus Lc for three values of the clearance into the peripheral compartment Cldα (α = 0.001, α = 1 and α = 100), whilst reverse clearance, from the peripheral compartment into the central compartment is fixed. As predicted by Eq. (40), the potency Lc; 50decreases as α increases and hence when kcp decreases. Of course, this is understandable: When transport to the peripheral compartment becomes easier, drug reaches its target more easily, less of it is required to achieve the same effect and the in vivo potency increases.
Sensitivity graphs of R and RLpversus Lc, on a semi-logarithmic scale, with regard to the clearance rate from the central to the peripheral compartment αCld where α = 0.1,1,10 and the clearance rate from peripheral to central compartment βCd is fixed (β = 1). Other parameters are listed in Table I
Left: Schematic illustrations of the consequences of two subjects with same the baseline target concentration (R0,A = R0,B), but different target turnover rates and losses. Right: Relationships between ligand concentration and normalised target occupancy when target baseline concentration is similar, but fractional turnover rates are different
Comparing the graphs of the concentration of the ligand-target complex RLpversus the ligand concentration in the central compartment Lc in Figs. 2 and 9, the latter, when target is located peripherally, shows up to be (i) asymmetrical and (ii) to exhibit a shift between the central and peripheral concentrations.
Discussion and Conclusions
Equations (32), (37), (38) and (39) summarise what is needed to apply and explain target R, ligand-target complex RLp and ligand L (Lc and Lp) interactions when the target is peripherally located. The effect of the permeability of the membrane between central and peripheral compartment and the volumes of these compartments show up explicitly in the expression for the in vivo potency given in Eq. (39) in combination with target turnover and ligand-target binding properties. This explicit expressions make it possible to give quantitative estimates.
DISCUSSION AND CONCLUSIONS
In Vivo Potency—the Role of Target Dynamics
The new concept of potency discussed in this paper departs from the previous one, based on the assumption that the actual expression level of target rather than its turnover rate will determine the potency. Thus, looking at the data of two individuals with the same target expression level (concentration), one would assume that the two individuals would require the same drug exposure. In these papers, we have shown that in fact, this need not be true. Instead, according to the definition of L50, the subject with the higher target elimination rate (kdeg) will need more drug compared to a subject with a slow target turnover rate, whilst the subject with the higher internalisation rate (ke(RL)) will require less drug.
Expressed mathematically, we demonstrated how the potency L50 is given in open as opposed to closed systems by the definitions
When target baseline levels are the same in two subjects, i.e. R0; A = R0; B, but one subject, say B as in Fig. 10 has a higher synthesis rate than A, i.e. ksyn; B > ksyn; A, the potency of drug in subject B will be numerically higher than in subject A, because kdeg; B > kdeg; A.
Non-symmetric Drug Distribution Between Central and Peripheral Compartment
We have seen that when target is located in the peripheral rather than the central compartment, the in vivo potency Lc; 50 will depend in the distributional rates between the two compartments, especially when they are not equal. Indeed, if Cldα denotes clearance out of the central compartment and Cldβ clearance into the central compartment, then, we have shown that if the synthesis rate of target ksyn is small, the in vivo potency with respect to the ligand concentration in the central compartment Lc; 50 and the in vivo potency with respect to the peripheral compartment Lp; 50 are related by the simple formula
Thus, if Cldα > Cldβ relatively easily and high receptor occupancy will be reached for lower ligand concentrations in the central compartment, i.e. L50c will be relatively small.
On the other hand, when Cldα < Cldβ ligand has difficulty reaching the target and the potency, L50; c will now be larger.
We make two observations about the three graphs in Fig. 10.
-
1.
The graphs appear to be translations of one another with a constant shift.
-
2.
All three graphs have a larger radius of curvature for lower values of RLp and a smaller radius of curvature for higher values of RLp.
As regards the first observation, it follows from Eq. (47) that
so that the graph shifts by log (Cldβ/Cldα) as we move from one curve to the next in Fig. 10.
Overall Conclusions
This analysis has focused on the necessity of using an open systems approach for assessment of in vivo pharmacological data.
The major difference between potencies of closed and open systems is that the expression of the latter (L50 in Eq. (3)) shows that target turnover rate (kdeg) rather than target concentration (R0) will determine drug potency. The efficacy (typically denoted Emax/Imax) of a ligand is, on the other hand, dependent on both target concentration and target turnover rate. When target is located peripherally, the ratio of inter-compartmental distribution (CLdα/CLdβ) impacts the potency derived for a centrally located target.
Derived expressions are practically and conceptually applicable when interpreting data translation across individuals, species and studies are done, and also for communication of results to a biological audience.
References
Gabrielsson J, Peletier LA. Pharmacokinetic steady-states highlight interesting target-mediated disposition properties. AAPS J. 2017;19(3):772–86.
Gabrielsson J, Peletier LA, Hjorth S. In vivo potency revisited—keep the target in sight. Pharmacol Ther. 2017;184:177–88. https://doi.org/10.1016/j.pharmthera.2017.10.011.
Wagner JG. Simple model to explain effects of plasma protein binding and tissue binding on calculated volumes of distribution, apparent elimination rate constants and clearances. Eur J Clin Pharmacol. 1976;10:425–32.
Sugiyama Y, Hanano M. Receptor-mediated transport of peptide hormones and its importance in the overall hormone disposition in the body. Pharm Res. 1989;6(3):192–202.
Levy G. Pharmacologic target mediated drug disposition. Clin Pharmacol Ther. 1994;56:248–52.
Michaelis L, Menten ML. Die Kinetik der Invertinwirkung Biochem Z. 1913;49:333–69.
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Phamacodyn. 2001;28(6):507–32.
Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22:1589–96.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35(5):573–91.
Peletier LA, Gabrielsson J. Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification. J Pharmacokinet Pharmacodyn. 2012;39:429–51.
Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn. 2010;37:323–46.
Yan X, Chen Y, Krzyzanski W. Methods of solving rapid binding target-mediated drug disposition model for two drugs competing for the same receptor. J Pharmacokinet Pharmacodyn. 2012;39:543–60.
Krippendorff BF, Kuester K, Kloft C, Huisinga W. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn. 2009;36:239–60.
Black JF, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220:141–62.
Kenakin T. Pharmacologic analysis of drug-receptor interaction. second ed. New York: Raven Press; 1993.
Kenakin T. A pharmacology primer: theory, applications, and methods. London: Elsevier Academic Press; 2015.
Neubig RR, Spedding M, Kenakin T, Christopoulos A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev. 2003;55:597–606.
Cao Y, Jusko WJ. Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2014;41:375–87.
Pang KS, Rodrigues AD, Peter RM, editors. Enzyme- and transporter-based drug-drug interactions: progress and future challenges. New York: Springer; 2010.
Pang KS, Huadong S, Chow ECY. Chapter 5. Impact of physiological determinants: flow, binding, transporters and enzymes on organ and total body clearances. In: Pang KS, Rodrigues AD, Peter RM, editors. Enzyme- and transporter-based drug-drug interactions: progress and future challenges. New York: Springer; 2010. p. 107–47. Ê.
Gabrielsson J, Weiner D. Pharmacokinetic and pharmacodynamic data analysis, Concept and applications 5th Ed. Apotekarsocieteten, Swedish Pharmaceutical Society, 2016.
Benson N, van der Graaf PH, Peletier LA. Cross-membrane signal transduction of receptor tyrosine kinases (RTKs): from systems biology to systems pharmacology. J Math Biol. 2013;66:719–42.
Benson N, Graaf PH, van der Peletier LA. Selecting optimal drug-intervention in a pathway involving receptor tyrosine kinases (RTKs). Nonlinear Anal. 2016;137:148–70.
Koch G, Jusko WJ, Schropp J. Target-mediated drug disposition with drug-drug interaction, part I: single drug case in alternative formulations. J Pharmacokinet Phamacodyn. 2017;44(1):17–26.
Koch G, Jusko WJ, Schropp J. Target mediated drug disposition with drug-drug interaction, part II: competitive and uncompetitive cases. J Pharmacokinet Phamacodyn. 2017;44(1):27–42.
Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs with two binding sites that bind to a target with one binding site. J Pharmacokinet Phamacodyn. 2016;44(5):463–75.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Appendix 1.1. Calculations for two targets
When ligand can bind two receptors, R1 and R2, the way the steady-state concentrations of the two complexes R1L and R2L depend on the ligand concentration L can be derived in a manner which very similar to the one used when only one receptor is present. Thus, we deduce from the steady-state equations for R1 and R2 in the system (6) that
where we have written \( {X}_i={k}_{e\left({R}_iL\right)}{R}_iL \) (i = 1, 2). Putting the expression for R1 into the right-hand side of the equation for dR1L/dt in Eq. (6), and equating it to zero, we obtain
Solving this equation for X1 yields
from which we obtain for R1L and R1:
and
the desired expressions given in Eq. (11).
Those for R2L and R2 are derived in a similar fashion.
Appendix 1.2. Calculations for two ligands
When two ligands can bind a single receptor, the dynamics is described by the system (Eq. (13)), which yields the following balance equation for ligand at steady state (cf. Eq. (16)):
As before, we can express R in terms of RL1 and RL2:
where we now write \( {Y}_i={k}_{e\left(R{L}_i\right)}R{L}_i \) (i = 1, 2).
We substitute this expression for R into the right-hand sides of each of the last two equations of the full system (Eq. (13)). Then, we obtain from the one but last equation in Eq. (13):
and for the last equation of Eq. (13):
Equations (A.7) and (A.8) are linear in Y1 and Y2 and can be solved explicitly. Their solution is
where
Writing Ai = Li/L50; i (i = 1, 2), the expressions for Y1 and Y2 in (A.10) yield the following relations between RLi and Li:
where for i = 1, 2,
Remark
In the expression for RL1, one can interpret the term (θ · L2 + L50; 1) as a “potency” related to L1, and in the expression for RL2, the term (θ−1 · L1 + L50; 2) can be viewed as a “potency” related to L2.
Appendix 1.3. Calculations when target is in the peripheral compartment
The four steady-state concentrations Lc, Lp, R and RLp solve the following set of four algebraic equations:
where we recall from “TARGET IN THE PERIPHERAL COMPARTMENT” section that
We now proceed in two steps: (i) We derive a relation between the concentrations of complex and ligand in the peripheral compartment, and then (ii) we derive a comparable relation but between concentrations of the complex in the peripheral compartment RLp and ligand in the central compartment Lc.
RLp in terms of Lp
The first equation of Eq. (A.13) yields a relation between the ligand concentrations in the two compartments:
Thus,
We use this expression to eliminate Lc from the second equation in Eq. (A.13) and so reduce the system to
Adding the first and μ times the third equation of Eq. (A.18), we obtain
and adding the second and the third equation yields
We use (A.19) in the fourth equation of Eq. (A.13). Dividing by kon and multiplying by kdeg yields
When we now divide by ke(RL) and rearrange the terms, we obtain
Note that this expression for Lp; 50 is the same as the one for L50 in Eq. (3).
RLp in terms of Lc
Whereas in the previous part of Appendix 1.3 we eliminated Lc, R and kinfus, we now eliminate Lp, R and kinfus. In fact, as before, kinfus is eliminated by means of Eq. (A.16).
-
(i)
Adding the second and the fourth equation of Eq. (A.13), we obtain an expression for Lp in therms of Lc and RLp:
and, as before,
Finally, we put the expressions for Lp and for R into the fourth equation of Eq. (A.13) and obtain, after division by kon,
where X = ke(RL)RLp. Therefore,
or
Equation (A.25) provides an expression for Lc as a function of X, i.e. of RLp. Below, we invert this expression and derive a formula for RLp as a function of Lc by multiplying Eq. (A.25) by (ksyn − X) and so obtain the quadratic equation for X:
where
This is a quadratic equation in X with roots
Because, we need the root which vanishes when Lc = 0, i.e. we need X−. Thus,
Using Eq. (A.22), we deduce the corresponding target depression.
Appendix 2. Data
For completeness, we add here the data used in different simulations. Thus, in Fig. 2, we use the data from Peletier and Gabrielsson (10) given in Table I and we use them from Cao and Jusko (18) given in Table II.
The data that have been used in studying the competition between two targets for a single ligand in Fig. 4 have been chosen artificially in order to highlight differences binding coefficients, elimination rates and concentrations of the two targets. They are given in Table III.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Peletier, L.A., Gabrielsson, J. New Equilibrium Models of Drug-Receptor Interactions Derived from Target-Mediated Drug Disposition. AAPS J 20, 69 (2018). https://doi.org/10.1208/s12248-018-0221-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-018-0221-x