Abstract
Most of the carbon found in fruits at harvest is imported by the phloem. Imported carbon provide the material needed for the accumulation of sugars, organic acids, secondary compounds, in addition to the material needed for the synthesis of cell walls. The accumulation of sugars during fruit development influences not only sweetness but also various parameters controlling fruit composition (fruit “quality”). The accumulation of organic acids and sugar in grape berry flesh cells is a key process for berry development and ripening. The present review presents an update of the research on grape berry development, anatomical structure, sugar and acid metabolism, sugar transporters, and regulatory factors.
Similar content being viewed by others
Introduction
The grapevine (Vitis vinifera L.), as a prominent fruit crop, is cultivated extensively around the world, with a cultivation history extending over 11,000 years (Dong et al. 2023). Grape berries serve a wide range of activities centered around table grapes, raisins, juice, wine and spirits, catering to a diverse array of markets (Kuhn et al. 2014; Li et al. 2021). As of 2022, the total global vineyard surface area was approximately 7.28 million hectares (https://www.statista.com/statistics/240635/total-vineyard-areas-worldwide-and-in-europe). The revenue in the fresh fruits market, which includes grapes, is expected to be around US$ 726 billion in 2024, with a forecasted annual growth of 6.58% (CAGR 2024–2028) (https://www.statista.com/outlook/cmo/food/fruits-nuts/fresh-fruits/worldwide). The global wine market, which is a major segment of grape consumption, was valued at USD 326 billion and is expected to grow at a CAGR of 4.4% during 2021–2026 (https://www.mordorintelligence.com/industry-reports/grapes-market). Additionally, red wine market specifically is expected to grow to $136 billion in 2028 at a CAGR of 5.2% (https://finance.yahoo.com/news/red-wine-global-market-report-161300922.html). The grapes market itself is expected to reach USD 215 billion in 2024 and grow at a CAGR of 7.10% to reach USD 303.20 billion by 2029 (https://www.mordorintelligence.com/industry-reports/grapes-market). These figures pinpoint the major economic impact of grape berry production and use.
During ripening, the berries accumulate high concentration of hexoses (1.1 M) in the vacuoles of flesh cells (Shahood et al. 2020; Du et al. 2023). The sweetness of grape berries impacts directly the sensory quality of berries and wine (Conde et al. 2007; Yang et al. 2023; Jiang et al. 2024). Sugar accumulation in the berries is a finely tuned outcome of numerous physiological processes including photosynthesis in the leaves, long-distance transport in phloem and unloading in sink organs (Martínez-Esteso et al. 2011; Lecourieux et al. 2014; Castellarin et al. 2016; Zhang et al. 2019; Martínez-Lüscher and Kurtural 2023). Till the eighties, in some wine areas, it was not uncommon to add sugars to the must (chaptalization) under controlled practices when the berry sugar content was not high enough to produce wines. Due to global climate change, it is now not uncommon to use sugar removing or de-alcoolization techniques because the grape berry sugar content becomes too high. A comprehensive understanding of sugar and acid accumulation and metabolism is crucial both for the selection and cultivation of superior grapevine varieties and for the optimization of agricultural practices aimed at enhancing fruit quality.
The sensitivity of grape leaves photosynthesis to various environmental factors (water, light and temperature) has been extensively studied (Jackson and Lombard 1993; Kolb et al. 2001; Hendrickson et al. 2004; Roig-Oliver et al. 2020; Rafique et al. 2023). However, it has been shown that the main driver of sugar accumulation in grape berries lies in the unloading process rather than in the ability of source leaves to synthesize and export photosynthetic sugars (Li et al. 2021). It involves two pathways that are not mutually exclusive: symplastic and apoplastic (Ruan et al. 2001; Viola et al. 2001; Zhang et al. 2006; Nie et al. 2010; Braun et al. 2014; Ren et al. 2023). Symplastic unloading through the plasmodesmata predominate during the early and mid-stages of grape berry development, while apoplastic unloading through the membranes becomes prominent at véraison (Zhang et al. 2022; Zhou et al. 2023). The plasmodesmata play a significant role in the switch from symplastic to apoplastic pathways (Zhang et al. 2006; Li et al. 2021; Zhou et al. 2023).
This switch is pivotal for the regulation of sugar accumulation (Zhang et al. 2006; Zhou et al. 2023). In addition to changes in plasmodesmatal density and permeability, it involves various enzymes of sugar metabolism, sugar transporter proteins and transcriptional regulators (Lecourieux et al. 2014; Durán-Soria et al. 2020; Li et al. 2021; Zenoni et al. 2023). Although this topic has been revised several times, significant and recent progress makes it useful to update it (Zhou et al. 2023; Liang et al. 2023). The present review highlights the challenges faced and future prospects, aiming to provide reference for in-depth studies into carbon accumulation in grape berries and thereby accelerate the breeding of high-quality grapes.
Grape berry development and anatomical structure
The development and ripening of grape berries are commonly divided into three stages corresponding to different balances in sugars, acids, and phenolic compounds. The initial growth Stage I after fruit set is characterized by rapid cell division and expansion, resulting in an increase in berry size. During this stage, the berries are hard and green, with high acid and low sugar content (Harris et al. 1968; Kuhn et al. 2014). Stage II is a lag phase, characterized by slow growth. At the end of this stage, major physiological changes lead to véraison (onset of ripening) is visually marked by a change in berry color. From this time on, Stage III (ripening) is characterized by a massive hexose accumulation of hexoses, berry softening, and synthesis of secondary metabolites, including aromatic compounds (Coombe 1992; Lu et al. 2023).
Each berry is composed of the exocarp which provides color and contains aromatic compounds and tannins, the mesocarp which is the main reservoir of sugars and acids, the seeds which contribute tannins and oils, and the endocarp as the thin layer that delineates the boundary of the locular cavities where the seeds develop (Fig. 1) (Coombe 1987; Conde et al. 2007; Fontes et al. 2011; Candar 2023). The vascular system interconnects these parts, ensuring the transport of the compounds that allow fruit development (Zhang et al. 2006). During the three developmental stages, the berries undergo major biochemical transformations, including sucrose-hexoses conversion, a change in the acid/sugar balance, the synthesis of tannins and aromatic compounds, which collectively contribute to their sensory attributes (Conde et al. 2007; Martínez-Esteso et al. 2011; Kuhn et al. 2014; Perotti et al. 2023).
Sugar and organic metabolism in grape berries
Grape berry metabolism involves a highly orchestrated interplay between sugar and acid biosynthesis, heavily reliant on photosynthetic carbon sources from the leaves (Sweetman et al. 2009). Sucrose, the main photoassimilate synthesized in the leaves, is translocated to the berries, forming the backbone for the synthesis of sugars and acids. The metabolism of sugars and organic acids undergo dramatic shifts at the véraison stage (Brady 1987; Giovannoni 2001, 2004; Maria et al. 2011; Giovannoni et al. 2017; Liu et al. 2023).
Before véraison, the berry engages in cell division and growth, accumulating organic acids, primarily malic acid, while sugar concentration remains at a low level (Conde et al. 2007; Dai et al. 2013; Etienne et al. 2013; Batista-Silva et al. 2018). At this stage, Sucrose is actively unloaded to berries and subsequently hydrolyzed by cell wall invertases (CWINV) into glucose and fructose (Maria et al. 2011; Kuhn et al. 2014,). After uptake by the flesh cells, glucose is further metabolized to phosphoenolpyruvate (PEP) by glycolysis. PEP lies at a critical crossroad leading to two separate pathways towards malate synthesis (Sweetman et al. 2009). PEP carboxylase (PEPC) catalyzes the conversion of PEP to oxaloacetate (OAA), which is then reduced to malate by NAD-dependent malate dehydrogenase (NAD-MDH) in the cytoplasm (Givan 1999). Alternatively, PEP may be converted by pyruvate kinase (PK) to form pyruvate, which can be further reduced to malate by NADP-dependent malic enzyme (NADP-ME) (Farineau and Lavalmartin 1977; Taureilles-Saurel et al. 1995; Sweetman et al. 2009; Martínez-Esteso et al. 2011). Then the malate can be transported into the mitochondrial matrix by malate transporter embedded in the inner mitochondrial membrane. Once inside, a mitochondrial NAD-dependent malate dehydrogenase converts malate to OAA and NADH, or a NAD-dependent malic enzyme converts it to pyruvate, CO2, and NADH (Sweetman et al. 2009). These intermediates feed the tricarboxylic acid (TCA) cycle, with the potential for malate regeneration depending on the metabolic flux within the mitochondria (Beriashvili and Beriashvili 1996; Ollat and Gaudillère 1997; Hanning et al. 1999). Excess malate is ultimately transported into the vacuoles, a process critical for maintaining the cytosolic pH balance and regulating the acid taste of the berry (Martínez-Esteso et al. 2011).
Grape berries exhibit a remarkable ability to synthesize and accumulate malate at pre-véraison stage, not only through the import of photosynthetically fixed carbon from the leaves, but also through the photosynthetic activity of exocarp cells (Sweetman et al. 2009; Garrido et al. 2023). Despite the limited presence of stomata in the berry skin, respiratory CO2 contributes to the synthesis of malate in flesh cells. Respiratory CO2 is converted to bicarbonate ion (HCO3 −) by carbonic anhydrase within the cytoplasm (Blanke and Lenz 1989; Garrido et al. 2023,). Phosphoenolpyruvate carboxylase (PEPC) then catalyzes the formation of oxaloacetate (OAA) from HCO3 − and the formation of phosphoenolpyruvate (PEP) in an irreversible β-carboxylation reaction (Beriashvili and Beriashvili 1996; Sweetman et al. 2009). The OAA is subsequently reduced by NAD-MDH to form malate. The malate is not a metabolic end point; it can be shuttled into chloroplasts where it undergoes decarboxylation by NADP-ME (Maria et al. 2011; Garrido et al. 2023). This reaction releases CO2 which can be re-assimilated by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in the Calvin-Benson-Bassham (CBB) cycle (Conde et al. 2007). The pyruvate resulting from this decarboxylation can be converted back to PEP by pyruvate, phosphate dikinase (PPDK), resulting in a regenerative loop within carbon metabolism (Ruffner 1982; Sweetman et al. 2009; Etienne et al. 2013; Garrido et al. 2023). The interconversion of pyruvate and malate provides connectivity to other essential metabolic pathways (Garrido et al. 2021). Both pyruvate and malate can feed the tricarboxylic acid (TCA) cycle, supporting cellular respiration and biosynthetic reactions (Fig. 3) (Etienne et al. 2013). Alternatively, malate can accumulate in the vacuole, contributing to the grape’s acidity, or it can serve as a substrate for gluconeogenesis, influencing sugar concentrations (Dai et al. 2013; Etienne et al. 2013; Reshef et al. 2022). Moreover, potassium influences the pH and acidity of grape must, with higher potassium levels often associated with lower acidity due to the interaction with malate in the berries (Rogiers et al. 2017).
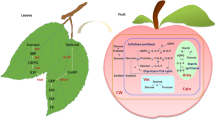
Sugar accumulation and sugar metabolism in the grape cells. PEP, phosphoenolpyruvate; OAA, oxaloacetic acid; CWINV, cell wall invertase; NINV, neutral invertase; VINV, vacuolar invertase; PEPC, phosphoenolpyruvate carboxylase; PK, pyruvate kinase; NAD-MDH, NAD-linked malic enzyme; NADP-ME, NADP-linked malic enzyme; FK, fructokinase; SS, sucrose synthase; SPS, sucrose phosphate synthase; SPP, sucrose phosphate phosphatase; HK, hexokinase; PFK, phosphofructokinase. CBB, Calvin-Benson-Bassham; TCA, tricarboxylic acid cycle
Post-véraison, there is an onset of hexose (glucose and fructose) accumulation and a concomitant decline in malate content (Davies and Robinson 1996). Sucrose metabolism is a central aspect of the biochemistry governing grape berry hexose accumulation (Ollat et al. 2002; Gambetta et al. 2010; Ruan 2014; Zhu et al. 2022). There is an overview of sugar metabolism in post-véraison berries (Fig. 2). At arrival in the berries, the sucrose transported by the phloem can be either hydrolyzed into glucose and fructose by invertases (INVs) or converted to UDPG and fructose by sucrose synthase (SS) (Li et al. 2012; Verma et al. 2011) (Fig. 2). Three types of invertases differ by their localization, cytosolic for the neutral invertase (NINV), vacuolar for the vacuolar invertase (VINV) and cell wall for the cell wall invertase (CWINV) (Ruan et al. 2010; Wang et al. 2014) (Fig. 2). The three types of invertase collectively ensure that hexose is available. SS provides an alternative route for sucrose degradation, generating fructose and UDP-glucose, which is particularly important for sustaining sucrose levels within cells (Verma et al. 2011). Hexokinase (HK) and fructokinase (FK) phosphorylate glucose and fructose to glucose-6-phosphate (G6P) and fructose-6-phosphate (F6P), respectively (Jang et al. 1997; Granot et al. 2013) (Fig. 2). Phosphofructokinase (PFK) then acts on F6P converting it to fructose-1,6-bisphosphate (F1,6BP), channeling it into glycolysis and subsequently into the TCA cycle, a key energy-producing pathway in respiration (Ronimus and Morgan 2001) (Fig. 2). Sucrose phosphate synthase (SPS) and sucrose phosphate phosphatase (SPP) cooperate in the resynthesis of sucrose, reutilizing the products of SS activity to regenerate sucrose from UDP-glucose and F6P (Huber and Huber 1996; Tian et al. 2012; Xia et al. 2021; Huang et al. 2022) (Fig. 2). This cycle, called “futile sucrose recycle” is not merely a metabolic detour but serves a regulatory function in balancing cellular energy and carbon partitioning, which is crucial during the stages of rapid growth and sugar accumulation of berries (Nguyen-Quoc and Foyer 2001).
The hypothetical model of sugar transporters involved in sugar accumulation in the grape berries. MC, mesophyll cell; PC, parenchyma cell; CC, Companion cell; CBB, calvin-benson-bassham; CWINV/Inv-CW, cell wall invertase; VINV, vacuolar invertase; NINV, neutral invertase; SUT, sucrose transporter; SWEET, sugars will eventually be exported transporter; VvHT, hexose transporter; VvTMT, tonoplast monosaccharide transporter
The metabolism of sucrose in grape berries is a multifaceted process that involves several specialized enzymes operating in concert across different cellular locations. The coordinated activities of SUTs, INVs, and SS manage the distribution and conversion of sucrose, while the activities of HK, FK, PFK, SPS, and SPP ensure its utilization and recycling within the cellular environment. The ‘futile sucrose cycle’ plays a substantial role in the developmental process, ensuring the hexoses accumulation essential for fruit quality (Nguyen-Quoc and Foyer 2001). Understanding the interplay of these enzymes and their regulation factors provide critical insight for strategies aimed at optimizing sugar content in grape berries, which is paramount for achieving desired wine attributes. Future research aimed at quantitatively measuring these enzyme activities in vivo and identifying their regulatory mechanisms offers the prospect of fine-tuning grape berry composition in the context of ever-changing environmental challenges and winemaking goals.
Sugar transporters in grape berries
Several families of sugar transporters play a key role in sugar accumulation during the ripening of grape berries. Therefore, these transporters are a key to understand the molecular mechanisms underlying these processes. The function of numerous sugar transport proteins has been elucidated (Reinders et al. 2012; Eom et al. 2015; Nino-Gonzalez et al. 2019; Wen et al. 2022; Pegler et al. 2023). Among these, three primary sugar transporter families have been identified as key contributors to sugar accumulation in plants: the Monosaccharide Transporters (MST), the Sucrose Transporters (SUT/SUC), and the Sugar Will Eventually be Exported Transporters (SWEET) protein families (Doidy et al. 2012; Pegler et al. 2023).
Members of the monosaccharide transporter (MST) family are ubiquitously distributed across plant species and are predicted to possess 12 transmembrane domains (Pao et al. 1998; Buttner and Sauer 2000; Buttner 2007). Within this family, seven subfamilies have been classified: Sugar Transport Proteins/Hexose Transporters (STP/HT), Tonoplast Sugar Transporters (TST, formerly known as TMT), Early Responsive to Dehydration Like 6 (ERDL6), Plastidic Glucose Transporters (pGlcT), Inositol Transporters (INT), Polyol/Monosaccharide Transporters (PMT, formerly known as PLT), and Vacuolar Glucose Transporters (VGT) (Buttner 2007; Slewinski 2011; Nino-Gonzalez et al. 2019). Notably, the STP/HT and TST subfamilies have been extensively studied.
MSTs have been acknowledged as pivotal in sugar accumulation (Fontes et al. 2011). In grapevine, a total of 59 monosaccharide transporter genes have been identified, which can be categorized into 7 subfamilies. It includes 20 VvHT (Subfamily I), 3 VvTMT (Subfamily II), 5 VvPMT (Subfamily III), 22 VvERDL6 (Subfamily IV), 2 VvVGT (Subfamily V), 3 VvINT (Subfamily VI), and 4 VvpGlcT/VvSGB1 (Subfamily VII) (Afoufa-Bastien et al. 2010). During berry development, the transcript levels of VvHT3 and VvHT6 are significantly higher than VvHT1, VvHT2, VvHT4 and VvHT5. (Hayes et al. 2007; Afoufa-Bastien et al. 2010). The VvHT1, VvHT2, VvHT4 and VvHT5 are most lowly expressed through the grape berry development period (Hayes et al. 2007; Afoufa-Bastien et al. 2010). Notably, the expression of VvHT3 is reduced at véraison but elevated highly in pre-véraison and post-véraison (Hayes et al. 2007). The expression of VvHT1 is strong shortly after anthesis but decreased during the period of rapid sugar accumulation (Hayes et al. 2007). VvHT6 expression remained high throughout the ripening process (Afoufa-Bastien et al. 2010). Immunofluorescence, immunolabeling and GFP fusion protein experiments revealed the plasma membrane localization of VvHT1, VvHT4, and VvHT5. VvHT1 exhibited higher glucose affinity and broader substrate specificity than VvHT4 and VvHT5, recognizing both D-glucose and D-fructose. VvHT3 was not capable of importing any sugar in mutant yeast strains (Vignault et al. 2005; Conde et al. 2006; Hayes et al. 2007). VvHT2 and VvHT6/VvTMT2 appear to be localized to the tonoplast, with VvHT6/VvTMT2 showing high sequence similarity to AtTMT2 (Agasse et al. 2009; Afoufa-Bastien et al. 2010). VvTMT1 and VvTMT2 exhibit higher expression levels in berries (Afoufa-Bastien et al. 2010). VvTMT2 is notably high expressed at the onset of ripening and post-véraison stages in V. vinifera ‘Sultanine’ berries (Cakir et al. 2012). The fusion expression of VvTMT1-GFP in yeast demonstrated tonoplast localization, and VvTMT1 glucose uptake was heterologously assessed by yeast hexose transporter mutants (Zeng et al. 2011). The various localization and affinity for substrate among these monosaccharide transporters suggest that their functions of sugar transport are diverse.
Reinders reported that AtSUTs can be divided into three types: Type I, which includes AtSUC1, 2, 5, 6, 7, 8, and 9; Type II includes AtSUC3; Type III includes AtSUC4 (Reinders et al. 2012; Wen et al. 2022). SUT/SUC transporters primarily transport sucrose into the SE-CC complex (Scofield et al. 2007; Slewinski et al. 2009, 2010; Wang et al. 2021). Expression of AtSUC2 can enhance sucrose loading in rice, thereby resulting in larger grains and improved crop yield (Wang et al. 2015). Suppressing tomato SUT1 (Hackel et al. 2006), knocking out rice SUT1 (Wang et al. 2021), and expressing SUT1 in pea (Lu et al. 2020), have indicated that SUT/SUC class transporters are crucial for phloem loading. Arabidopsis AtSUC5 enables sucrose inflow into the endosperm, ultimately providing nutrition to the embryo (Baud et al. 2005). In seeds, OsSUT1/3/4, localized to the starchy layer, can transport sucrose into seeds to enhance sucrose unloading (Furbank et al. 2001; Bai et al. 2016). Sugarcane SUT5 and SUT6 are highly expressed in source leaves, aiding phloem loading (Zhang et al. 2016), SUT1 does not participate in phloem unloading but is involved in recycling sucrose leaked into the apoplast back to the vascular parenchyma cells (Glassop et al. 2017). Maize SUC4 is localized to the tonoplast and can export sucrose from vacuoles (Carpaneto et al. 2010; Schneider et al. 2012). Furthermore, AtSUC5 can also transport biotin (Ludwig et al. 2000), and AtSUC9 is able to transport a wide range of glucosides (Sivitz et al. 2007).
VvSUTs (VvSUC2, VvSUC11, VvSUC12, and VvSUC27) in different Vitis varieties focus on the expression, localization, function and regulation. VvSUC2 exhibits low expression levels or not detected across various tissues and organs. VvSUC27 is ubiquitously expressed in vegetative organs while is weakly expressed in berries (Afoufa-Bastien et al. 2010). The expression of VvSUC11 and VvSUC12 are relatively low in berries but stays stable during the ripening stages (Afoufa-Bastien et al. 2010). VvSUC12 and VvSUC27 were also expressed in seeds but at a lower level (Afoufa-Bastien et al. 2010). VvSUC11 and VvSUC12 with high-affinity/low-capacity to sucrose, control sugar distribution. VvSUC11, VvSUC12, and VvSUC27 can form homodimers and heterooligomers to guide the rapid transport of sucrose in SE (Cai et al. 2021). VvSUC27 is localized on the plasma membrane. Overexpressing VvSUCs (VvSUC11 or VvSUC12 or VvSUC27) in tobacco and Arabidopsis showed that the plants grew faster, had increased yield, and enhanced stress resistance (Cai et al. 2017, 2020). Similarly, SUTs in grape “Zuoshan-1” responded to various stresses, promoting sucrose metabolism and hormone synthesis (Cai et al. 2019). However, the research of VvSUTs function is still predominantly conducted in heterologous systems, such as Arabidopsis, tobacco. In fact, a direct assessment of their roles in sugar accumulation in grape berries is limited or almost non-existent. This gap highlights the need for more research in grape berries to fully understand the contributions of VvSUTs in sugar accumulation and ripening.
SWEETs are a novel transporter family in plants involved in cellular sugar efflux (Chen et al. 2010), primarily transporting substrates such as glucose, fructose, and sucrose (Chardon et al. 2013; Klemens et al. 2013; Eom et al. 2015). In angiosperms, there are an average of 20 SWEET family members, which are differentially expressed across diverse tissues and organs. In Arabidopsis, SWEET members are phylogenetically divided into four clades, with Clade I (SWEET1-2), Clade II (SWEET3-8), and Clade IV (SWEET16-17) mainly transporting monosaccharides, whereas Clade III (SWEET9-15) mainly transports sucrose (Chen et al. 2010, 2015). SWEET transporters can be localized in various subcellular compartments: SWEET1, 8, 9, 11, 12, and 15 are primarily localized to the plasma membrane (Seo et al. 2011; Kryvoruchko et al. 2016), SWEET2, 16, and 17 to the tonoplast (Chardon et al. 2013; Klemens et al. 2013; Guo et al. 2014; Chen et al. 2015), and SWEET9 to the Golgi membrane (Lin et al. 2014; Chen et al. 2015). SWEET proteins are involved in various functions including plant carbon partitioning, pollen nutrition supply, seed development, organ senescence, hormone transport and interactions between plants and pathogens (Chen et al. 2015; Hutin et al. 2015; Ho et al. 2019; Ni et al. 2020; Braun 2022; Xue et al. 2022; Radchuk et al. 2023). As research continues to deepen, the regulatory networks of SWEET proteins and their potential in improving crop yield and stress resistance are expected to be more comprehensively assessed and utilized.
In grapevine, there are 17 SWEET homologues, among which among which VvSWEETs (VvSWEET1, VvSWEET2a, VvSWEET2b, VvSWEET4, VvSWEET7, VvSWEET10, VvSWEET15 and VvSWEET17a) have been identified as being expressed during grape berries development. Among them, VvSWEET1, VvSWEET2a, VvSWEET2b, VvSWEET10, VvSWEET15, and VvSWEET17a displayed higher expression in Chardonnay berries than those in other organs (Zhang et al. 2019). VvSWEET10 is highly expressed in véraison (Zhang et al. 2019). Specifically, VvSWEET15 is strongly expressed in both véraison and post-véraison in Chardonnay berries and the expression level is much higher than that of VvSWEETs (VvSWEET1, VvSWEET2a, VvSWEET2b, VvSWEET10, VvSWEET15 and VvSWEET17a) (Zhang et al. 2019). VvSWEET10, a plasma membrane transporter, was found to significantly increase glucose, fructose, and total sugar content when overexpressed in grape callus and tomato (Zhang et al. 2019). VvSWEET15 was highly expressed in the three grape varieties and was positively correlated with the hexose content during ripening (Ren et al. 2020). In our research, VvSWEET10 and VvSWEET15 exhibit notably high expression level in grape berry and an in-depth gene-silencing and overexpressing studies of VvSWEET15 demonstrate that VvSWEET15 facilitates hexose accumulation at post-véraison stages (unpublished data). Future research is required to explore these potential roles and deepen understanding of the molecular mechanisms underlying grape ripening and sweetness. VvSWEET4 is lowly expressed in small green berry and pre-version green berry, but is highly expressed in post-version berry of V. vinifera 40,024 (Chong et al. 2014). VvSWEET4 is a glucose transporter located on the plasma membrane (Chong et al. 2014). Overexpression of VvSWEET4 in grapevine root hairs led to increased glucose content in the root hairs, upregulation of genes in the flavonoid biosynthetic pathway, and enhanced resistance to soil pathogen infection (Meteier et al. 2019). VvSWEET7 is highly expressed during both the green berry phase and ripening phase in Trincadeira grapes and is able to transport monosaccharides, disaccharides, and polyols (Breia et al. 2020). VvSWEET7 may participate in plant defense by rapidly removing pathogen-synthesized mannitol from the extracellular space (Breia et al. 2020). The expression of VvSWEET2a, VvSWEET7, and VvSWEET15 increases significantly when grapes are infected by Botrytis cinerea, whereas different developmental stages of infection downregulate the expression of VvSWEET10, 11, 17a, and 17d (Breia et al. 2020).
Sugar transporters present a complex, critical network essential for sugar accumulation in grape berries. The precise expression patterns of these transporters—coordinated with development stages, specificity for sugar substrates, and cellular localization—reflect the intricate control of sugar distribution within the berry. The current understanding of sugar transport proteins in grape berries is limited due to restrictions in transgenic systems, leaving their exact functions somewhat unclear. However, to enhance our knowledge of the role of sugar transport proteins in sugar accumulation in grape berries, we have objectively organized the existing data into a hypothetical model (Fig. 3). Given that VvSUC12 has two structural features unique to the SUT2/SUC3 subfamily (including AtSUC3), which shares a 66.6% similarity with AtSUT3 and is expressed in mature grape leaves, it is hypothesized to be involved in loading sucrose into the phloem SE-CC complex, akin to AtSUT3’s function in sucrose funneling from the mesophyll towards the phloem (Meyer et al. 2000; Afoufa-Bastien et al. 2010) (Fig. 3). VvSWEET17a, with high expression in mature leaves, might function similarly to AtSWEET11 and 12, facilitating sucrose across the plasma membrane from mesophyll cells to the apoplastic space (Chen et al. 2012) (Fig. 3). This suggests that SWEET17a carries sucrose across the plasma membrane and VvSUT12 further move it into the phloem.
Subsequently, sucrose is transported long distances through the phloem and eventually reaches the unloading phloem. Here, it is speculated that sucrose is released into the apoplastic space through VvSUT11, VvSUT12, and VvSWEET10 (Fig. 3). The functions of VvSUT11 and VvSUT12 are similar to the sucrose efflux functions of homologs AtSUT4 (belongs to the SUT4 subfamily, including AtSUC4) and AtSUT3 respectively (Manning et al. 2001; Afoufa-Bastien et al. 2010). The function of SWEET10 in sucrose transport has been demonstrated in sucrose-deficient yeast (unpublished data from our laboratory) (Fig. 3). Most studies suggest that released sucrose is mainly translocated through symplastic transport via plasmodesmata and eventually accumulates hexoses via plasma membrane-located HTs, as well as vacuolar membrane transporters-located TMTs and HTs at pre-véraison (Breia et al. 2021; Braun et al. 2022; Wen et al. 2022; Ren et al. 2023). At this stage, the HTs are hypothesized to be VvHT3/VvHT7 and VvHT6, the TMTs are VvTMT1 and VvTMT2, according to their transcriptome data (Afoufa-Bastien et al. 2010; unpublished expressing data from our laboratory) (Fig. 3). After véraison, part of the released sucrose is degraded into hexoses by cell wall invertase (CWINV) and neutral invertase (NINV), which are then transported across the plasma membrane by VvHT6 and VvSWEET15, and across the vacuolar membrane by VvTMT1, VvTMT2, VvHT6, and VvSWEET15, ultimately accumulating hexoses in the vacuole (Hayes et al. 2007; Afoufa-Bastien et al. 2010; unpublished expressing data from our laboratory) (Fig. 3). This narrative reflects a hypothesis based on the current limited data and requires further research for confirmation.
Overall, the development of grape berries is characterized by the continuous accumulation of sugars, which forms many important carbohydrates in mature berries and ultimately dictates berry yield and quality (Smeekens 2000; Rolland et al. 2002). In grapevine, sugar is exported from source leaves and eventually accumulates in the FCs through symplastic and apoplastic pathways. Sucrose is synthesized in the photosynthetic mesophyll cells of leaves and loaded into phloem sieve tubes via symplastic or apoplastic loading pathways, which is propelled by hydrostatic pressure. After long-distance transport, sucrose is unloaded at the site of CCs and transported into the apoplastic space, subsequently either transported by VvSUTs on the plasma of PCs or cleaved into hexoses by CWINV or SS (Chen et al. 2017; Wan et al. 2018; Duan et al. 2020). These hexoses are later transported into the FCs through specific VvPMTs located on the plasma or vacuolar membranes (Grappadelli et al. 2019). These processes maintain a sucrose concentration gradient at unloading sites, ensuring rapid unloading and accumulation of hexose (Lecourieux et al. 2014). Research indicates that the phloem unloading pathway in berries undergoes a transition during berry development, shifting from symplastic to apoplastic unloading pathway around the time of véraison (Zhang et al. 2006). As grape berries develop into the post-véraison stage, the deposition of callose blocks plasmodesmata, resulting in the symplastic isolation of between CCs and PCs/FCs (Zhang et al. 2006). This isolation only allows sucrose to be unloaded through VvSUTs on the CC plasma membranes into the apoplastic space, where hexose ultimately transported into the FCs via apoplastic unloading pathways. Thus, sugar transporters and enzymes associated with sugar metabolism together form a complex regulatory network that governs sugar accumulation in grape berries. Understanding these intricate interactions between sugar transporters and various metabolic pathways enables researchers to devise strategies for manipulating sugar distribution and metabolism, which can enhance fruit quality and prolong shelf life.
Regulatory factors influencing sugar accumulation
Despite extensive research on the function of sugar transporters in various plant species, reports focusing on the transcriptional and post-translational regulation of these proteins are relatively scarce. The current knowledge predominantly addresses transcriptional regulation of a limited number of transporter genes and less is known about the post-translational control mechanisms that modulate transporter activity.
Sugar transporters at transcription level only concentrated on minority. For instance, in grapevine, the interaction between VvMSA and the VvHT1 promoter indicates a positive regulation of VvHT1 promoter activity (Çakir et al. 2003). The R2R3-type MYB96 transcription factor directly binds to the STP13 promoter activating its expression, which induces sugar uptake and enhances plant tolerance to adverse environmental challenges (Lee and Seo 2021). In watermelon, the transcription factor SUSIWM1 positively regulates ClTST2 gene, promoting the accumulation of sucrose, glucose, and fructose in the flesh cell vacuoles (Ren et al. 2018). In rice, NF-YB1 influences the expression of OsSUT1/3/4 located in the aleurone layer, intensifying sucrose unloading (Furbank et al. 2001; Bai et al. 2016). The OsDOF11 transcription factor binds to the promoter regions of OsSUT1, OsSWEET11, and OsSWEET14 enhancing the expression of these genes, thereby affecting sugar transport in rice. The mutant Osdof11 exhibits dwarfed stature, reduced tillering, insensitivity to sucrose-mediated root growth inhibition, reduced sugar accumulation in leaves, and diminished phloem sucrose flow. The ABA-responsive transcription factor OsbZIP72 can bind to the promoter regions of OsSWEET13 and OsSWEET15, activating their expression in response to drought stress (Mathan et al. 2021). In cotton, the transcription factor GhMYB212 binds to the GhSWEET12 promoter, promoting its expression to regulate the carbon supply required for cotton fiber elongation (Sun et al. 2019). Within pear fruit, PuWRKY31 directly binds to the PuSWEET15 promoter, upregulating its expression and enhancing high sucrose accumulation in the fruit of high-sugar bud sports (Li et al. 2020). The lily transcription factor LoABF2 (an AREB/ABF binding factor) can bind to the LoSWEET14 promoter, inducing LoSWEET14 expression and participating in the ABA signaling pathway to promote soluble sugar accumulation in response to various abiotic stresses (Zeng et al. 2022). The VvMYB15 transcription factor is implicated activating the expression of VvSWET15 (Li et al. 2022). In apple (Malus × domestica) variety “Gala”, MdWRKY9 which bound to the MdSWEET9b promoter interacted with MdbZIP23 (basic leucine zipper) and MdbZIP46, and upregulated MdSWEET9b expression, thereby influenced apple fruit sugar accumulation (Zhang et al. 2023).
Post-translational research on sugar transporters primarily focuses on control by kinases and phosphatases. For example, the expression of monosaccharide transporters (VvHT3, VvHT4, VvHT5, and VvHT6) in grapevine is regulated by protein kinases (VvSK1), modulating sugar intake and accumulation (Lecourieux et al. 2010). Glucose can inhibit the transcription of VvHT1 via a process dependent on hexokinase (HXK) and can reduce the abundance of VvHT1 protein in the plasma membrane through HXK-mediated post-translational modifications (Conde et al. 2006). In Arabidopsis, the wall-associated kinase AtWAKL8 acts as a positive regulator of AtSUC2, capable of phosphorylating AtSUC2 thereby enhancing its sucrose-binding capacity (Xu et al. 2020). The ethylene-responsive transcription factor MaRAP2-4 activates the expression of the Arabidopsis SWEET10, modulating sugar accumulation to increase waterlogging tolerance and enhance the drought and salt tolerance of the Lamiaceae species (Mentha arvensis) (Phukan et al. 2018). Additionally, the transport activity of sugar transporters can be regulated through interaction with binding proteins. In potato, the interaction between StSP6A and StSWEET11 prevents the leakage of sucrose into the apoplastic space during tuber development and leads to reduced transport activity of StSWEET11 when bound to StSP6A in protoplasts and yeast (Abelenda et al. 2019). Rice copper transporters (OsCOPT1 and OsCOPT5) interact with OsSWEET11 to modulate copper distribution during infection with Xoo, although it is not yet clear if this interaction affects the sugar transport of OsSWEET11 (Yuan et al. 2010).
The transcriptional and post-translational regulation of sugar transporters uncover a complex network dictating the functional state of these proteins. Transcription factors orchestrate the transcriptional response to developmental cues and environmental stimuli, while kinases and phosphatases finely tune transporter activity to adapt to cellular needs. As research progresses, elucidating the precise dynamic regulatory mechanisms will be crucial for a more comprehensive understanding of sugar transport in plants, especially in grapevine, with implications for agricultural productivity and stress resilience.
Environmental factors influencing sugar accumulation
Temperature poses significant threats to viticulture in current and future global climate change scenarios (Venios et al. 2020). Temperature significantly influences grapevine metabolism and consequently sugar accumulation in grapes. Warmer temperatures accelerate the rate of sugar accumulation (measured in Brix) by enhancing photosynthetic activity in leaves, which leads to increased sugar production and transport to the berries (Stanfield et al. 2024). However, the highest quality wine is produced when the berries simultaneously achieve optimal sugar-to-acid ratios and maximum levels of pigments, aromas, and flavors (Gladstones 2011). High temperatures accelerate sugar accumulation in grape berries, leading growers to harvest early to avoid producing overly sweet, flat-tasting wines with high alcohol content, although the berries have not yet reached optimal flavor development (Delrot et al. 2020). This creates a challenge for winemakers because the sugars and flavors contents develop at different rates. To address this issue, growers select grape cultivars from hotter wine regions that possess traits enhancing hydraulic resistance. This adaptation helps improve wine quality by slowing the rate of sugar accumulation (Stanfield et al. 2024).
Sunlight exposure plays a pivotal role in shaping the quality of grape bunches and berries, significantly affecting the physiological and metabolic pathways of grapevines and ultimately influencing sugar accumulation in grapes (Friedel et al. 2015). Increased sunlight exposure boosts photosynthesis rates, potentially enhancing sugar availability for berry development. Berries that are fully exposed to sunlight tend to have smaller diameters and higher total soluble solids (up to 22.4 Brix) with lower acidity and juice pH compared to those in partial or complete shade (Somkuwar et al. 2023). This exposure also increases levels of hydroxybenzoic acid, gallic acid, ellagic acid, and anthocyanins, while decreasing flavan-3-ols and amino acids compared to shaded berries (Downey et al. 2004; Somkuwar et al. 2023). In contrast, shaded bunches show higher proline concentrations, underlining the profound impact of sunlight on the biochemical composition and quality of grape berries (Moukarzel et al. 2023). Additionally, the temperature of berry skins, elevated by direct sunlight, affects enzymatic activities crucial for sugar metabolism. Sunlight also influences the expression of genes involved in sugar transport and metabolism, further impacting sugar accumulation (Moukarzel et al. 2023). However, excessive sunlight or heat can cause detrimental effects like berry sunburn and reduced photosynthetic efficiency, potentially diminishing sugar content of berries (Gambetta et al. 2021). Therefore, achieving optimal sunlight exposure through proper vineyard management practices such as leaf removal, shoot positioning, and vine spacing is essential for maximizing sugar content and enhancing grape quality, which are vital for the final quality of wine (Smart 1985; Palliotti et al. 2011; Reynolds 2022).
Genetic diversity of sugar accumulation in grape berries
Within the Vitis genus, there is considerable genetic variability in both sugar composition and concentration. the total sugar concentration, commonly quantified as total soluble solids (TSS), ranges from 18.7 to 27 Brix at maturity across 78 cultivars of Vitis vinifera, which includes both table grape and wine grape varieties (Kliewer 1967a). Kliewer found a broader variation among 26 Vitis species from North America and the Middle East, with TSS at maturity spanning from 13.7 Brix in V. champinii to 31.5 Brix in V. riparia from Wyoming (Kliewer 1967b). Furthermore, among 18 Eurasian grape species in Xinjiang region, the TSS at maturity have been reported to vary widely, from as low as14.9 Brix in Victoria to as high as 25.1 Brix in Summer Black (Zhong et al. 2023). In terms of specific sugar types, all cultivars primarily accumulate glucose and fructose, typically glucose content ranging from 42.13 to 46.80% of the total sugar and the fructose contents varied from 42.68 to 50.95%, while exhibiting very low levels of sucrose which varied from 6.17 to 12.69% (Zhong et al. 2023). Conversely, V. labrusca and V. rotundifolia, along with their interspecific hybrids, are noted for higher sucrose levels, ranging from 5 to 58.28 g/L, along with moderate concentrations of glucose and fructose (from 35 to 54 g/L), marking a distinct contrast in sugar profiles (Dai et al. 2011).
Cultivated V. vinifera has a significantly higher concentration of soluble sugars compared to wild Vitis species. To explore how gene variations related to sugar metabolism and transport contribute to the higher sugar accumulation in cultivated V. vinifera compared to wild Vitis species, the genomes of 14 V. vinifera and 13 wild species were resequenced (Xin et al. 2013). Eleven gene families pertinent to sugar metabolism, identified from the V. vinifera 12 X genome, included two families involved in sucrose synthesis: SPS and SPP. SPS synthesizes sucrose 6-phosphate using UDP-glucose and D-fructose 6-phosphate, which SPP then converts into sucrose. This sucrose is further processed into UDP-glucose and fructose by sucrose synthase (SUSy), and into glucose and fructose by invertase (INV). Invertases, categorized into three subfamilies based on their biochemical properties and subcellular locations, play key roles in these conversions (Sturm 1999; Nonis et al. 2008). Additionally, seven enzymes associated with glycolysis were identified, including FK, HK, and others (Xin et al. 2013). Alongside sugar transporter genes reported in grapes, 138 DNA regions on the V. vinifera genome were examined to assess the impact of domestication on sugar content in grapes (Afoufa-Bastien et al. 2010; Xin et al. 2013).
Rapid progress in DNA sequencing and genotyping has enabled more effective Whole Genome Amplification (WGA) studies, especially in species with sparse genetic data (Lijavetzky et al. 2007; Pindo et al. 2008; Xia et al. 2009; Lam et al. 2010; Dong et al. 2023). This has led to a significant increase in the identification of genetic variations like SNPs and InDels, which are crucial for understanding genetic diversity and relationships across different accessions (Myles et al. 2011). A recent study analyzed the genetic diversity of grapevine by resequencing genomic DNA from 27 V. vinifera and wild Vitis species, producing 46.9 Gb of DNA sequences (Xin et al. 2013). Despite a low alignment rate with the reference genome, possibly due to its incompleteness or the substantial genetic variation between the samples and the reference, the researchers identified thousands of SNPs and InDels that suggest significant genetic diversity and divergence due to domestication (Xin et al. 2013). They discovered genes involved in sugar metabolism that exhibited considerable differences in SNPs/InDels between wild and cultivated grapes, underscoring the role of these genes in grape berry development and sugar accumulation (Xin et al. 2013). This genetic exploration not only enhances our understanding of influence of artificial selection on grapevine genetics biological mechanisms underlying sugar accumulation but also provided insights into the evolutionary dynamics that continue to shape this species.
Conclusion
The journey from flowering to the harvest of sweet ripe grape berries results depends on the supply of sugars, on a complex interplay between acid and sugar metabolism, the efficiency of sugar transport systems, and regulatory factors orchestrating these processes (Lucas et al. 2013; Griesser et al. 2024). While considerable progress has unraveled various sugar metabolism pathways and function of the enzymes, the roles and regulation of sugar transport proteins (SUC, HT, TMT, SWEET) in diverse fruit crops, their cellular localization, and the exact operational dynamics of these proteins within fruit tissues largely remain elusive (Lecourieux et al. 2014; Li et al. 2021; Ren et al. 2023). Enhanced knowledge on these fronts bears the promise of paving the way for advancing grapevine cultivation, enology, and viticultural practices.
Availability of data and materials
Not applicable.
Abbreviations
- CBB:
-
Calvin-benson-bassham
- CWINV:
-
Cell wall invertase
- ERDL6:
-
Early responsive to dehydration like 6
- FK:
-
Fructokinase
- HK:
-
Hexokinase
- INT:
-
Inositol transporters
- INV:
-
Invertase
- MST:
-
Monosaccharide transporter
- NINV:
-
Neutral invertase
- NAD-MDH:
-
NAD-linked malic enzyme
- NADP-ME:
-
NADP-linked malic enzyme
- OAA:
-
Oxalacetic acid
- PEP:
-
Phosphoenolpyruvate
- PEPC:
-
Phosphoenolpyruvate carboxylase
- PFK:
-
Phosphofructokinase
- pGlcT:
-
Plastidic glucose transporter
- PK:
-
Pyruvate kinase
- PMT:
-
Polyol monosaccharide transporter
- SS:
-
Sucrose synthase
- SPS:
-
Sucrose phosphate synthase
- SPP:
-
Sucrose phosphate phosphatase
- STP:
-
Sugar transport protein
- SUT/SUC:
-
Sucrose transporter
- SUSy:
-
Sucrose synthase
- SWEET:
-
Sugars will eventually be exported transporter
- TCA:
-
Tricarboxylic acid cycle
- TST:
-
Tonoplast sugar transporter
- VGT:
-
Vacuolar glucose transporter
- VINV:
-
Vacuolar invertase
- VvHT:
-
Hexose transporter
- VvTMT:
-
Tonoplast monosaccharide transporter
- VvPMT:
-
Polyol/monosaccharide transporter
- VvVGT:
-
Vacuolar glucose transporter
- VvINT:
-
Inositol transporter
- WGA:
-
Whole Genome Amplification
References
Abelenda JA, Bergonzi S, Oortwijn M, Sonnewald S, Du M, Visser RG, et al. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr Biol. 2019;29(7):1178–86.
Afoufa-Bastien D, Medici A, Jeauffre J, Coutos-Thévenot P, Lemoine R, Atanassova R, et al. The Vitis vinifera sugar transporter gene family: phylogenetic overview and macroarray expression profiling. BMC Plant Biol. 2010;10:1–22.
Agasse A, Vignault C, Kappel C, Conde C, Gerós H, Delrot S. Sugar transport & sugar sensing in grape. In: Roubelakis-Angelakis KA, editor. Grapevine molecular physiology & biotechnology. Netherland: Springer; 2009. p. 105–39.
Bai AN, Lu XD, Li DQ, Liu JX, Liu CM. NF-YB1-regulated expression of sucrose transporters in aleurone facilitates sugar loading to rice endosperm. Cell Res. 2016;26(3):384–8.
Batista-Silva W, Nascimento VL, Medeiros DB, Nunes-Nesi A, Ribeiro DM, Zsögön A, et al. Modifications in organic acid profiles during fruit development and ripening: correlation or causation? Front Plant Sci. 2018;9: 1689.
Baud S, Wuilleme S, Lemoine R, Kronenberger J, Caboche M, Lepiniec L, et al. The AtSUC5 sucrose transporter specifically expressed in the endosperm is involved in early seed development in Arabidopsis. Plant J. 2005;43(6):824–36.
Beriashvili TV, Beriashvili LT. Metabolism of malic and tartaric acids in grape berries. Biochemistry. 1996;61(10):1316–21.
Blanke MM, Lenz F. Fruit photosynthesis. Plant Cell Environ. 1989;12(1):31–46.
Brady CJ. Fruit ripening. Ann Rev Plant Physiol. 1987;38:155–78.
Braun DM. Phloem loading and unloading of sucrose: what a long, strange trip from source to sink. Annu Rev Plant Biol. 2022;73:553–84.
Braun DM, Wang L, Ruan YL. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot. 2014;65(7):1713–35.
Breia R, Conde A, Pimentel D, Conde C, Fortes AM, Granell A, et al. VvSWEET7 is a mono-and disaccharide transporter up-regulated in response to Botrytis cinerea infection in grape berries. Front Plant Sci. 2020;10:1–13.
Breia R, Conde A, Badim H, Fortes AM, Geros H, Granell A. Plant SWEETs: from sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021;186(2):836–52.
Buttner M. The monosaccharide transporter(-like) gene family in Arabidopsis. Febs Lett. 2007;581(12):2318–24.
Buttner M, Sauer N. Monosaccharide transporters in plants: structure, function and physiology. BBA-Biomembranes. 2000;1465(1–2):263–74.
Cai YM, Tu WR, Zu Y, Jing Y, Xu Z, Lu J, et al. Overexpression of a grapevine sucrose transporter (VvSUC27) in tobacco improves plant growth rate in the presence of sucrose in vitro. Front Plant Sci. 2017;8:1–18.
Cai YM, Yan J, Li QK, Deng ZF, Liu SL, Lu J, et al. Sucrose transporters of resistant grapevine are involved in stress resistance. Plant Mol Biol. 2019;100:111–32.
Cai YM, Yan J, Tu WR, Deng ZF, Dong WJ, Gao H, et al. Expression of sucrose transporters from Vitis vinifera confer high yield and enhances drought resistance in Arabidopsis. Int J Mol Sci. 2020;21(7):1–17.
Cai YM, Yin L, Wang J, Dong WJ, Gao H, Xu JX, et al. Hetero/homo-complexes of sucrose transporters may be a subtle mode to regulate sucrose transportation in grape berries. Int J Mol Sci. 2021;22(21):12062.
Cakir B, Giachino RRA. VvTMT2 encodes a putative tonoplast monosaccharide transporter expressed during grape berry (Vitis vinifera Cv. Sultanine) ripening. Plant Omics. 2012;5:576–83.
Çakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R. A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell. 2003;15(9):2165–80.
Candar S. How abiotic stress induced by artificial wounding changes maturity levels and berry composition of Merlot (Vitis vinifera L.). Eur Food Res Technol. 2023;249(10):2611–23.
Carpaneto A, Koepsell H, Bamberg E, Hedrich R, Geiger D. Sucrose and H + dependent charge movements associated with the gating of sucrose transporter ZmSUT1. PLoS One. 2010;5(9):e12605.
Castellarin SD, Gambetta GA, Wada H, Krasnow MN, Cramer GR, Peterlunger E, et al. Characterization of major ripening events during softening in grape: turgor, sugar accumulation, abscisic acid metabolism, colour development, and their relationship with growth. J Exp Bot. 2016;67(3):709–22.
Chardon F, Bedu M, Calenge F, Klemens PAW, Spinner L, Clement G, et al. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr Biol. 2013;23(8):697–702.
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468(7323):527–32.
Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–11.
Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–94.
Chen C, Yuan Y, Zhang C, Li H, Ma F, Li M. Sucrose phloem unloading follows an apoplastic pathway with high sucrose synthase in Actinidia fruit. Plant Sci. 2017;255:40–50.
Chong J, Piron MC, Meyer S, Merdinoglu D, Bertsch C, Mestre P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J Exp Bot. 2014;65(22):6589–601.
Conde C, Agasse A, Glissant D, Tavares R, Gerós H, Delrot S. Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol. 2006;141(4):1563–77.
Conde C, Silva P, Fontes N, Dias ACP, Tavares RM, Sousa MJ, et al. Biochemical changes throughout grape berry development and fruit and wine quality. Food. 2007;1:1–22.
Coombe BG. Distribution of solutes within the developing grape berry in relation to its morphology. Am J Enol Vitic. 1987;38(2):120–7.
Coombe BG. Research on development and ripening of the grape berry. Am J Enol Vitic. 1992;43(1):101–10.
Dai ZW, Ollat N, Gomès E, Decroocq S, Tandonnet J-P, Bordenave L, et al. Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition: a review. Am J Enol Viticult. 2011;62(4):413–25.
Dai ZW, Léon C, Feil R, Lunn JE, Delrot S, Gomès E. Metabolic profiling reveals coordinated switches in primary carbohydrate metabolism in grape berry. J Exp Bot. 2013;64(5):1345–55.
Davies C, Robinson SP. Sugar accumulation in grape berries-cloning of two putative vacuolar invertase cDNAs and their expression in grapevine tissues. Plant Physiol. 1996;111(1):275–83.
Delrot S, Grimplet J, Carbonell-Bejerano P, Schwandner A, Bert P-F, Bavaresco L, et al. Genetic and genomic approaches for adaptation of grapevine to climate change. In: Genomic designing of climate-smart fruit crops. 2020. p. 157–270.
Doidy J, Grace E, Kuehn C, Simon-Plas F, Casieri L, Wipf D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012;17(7):413–22.
Dong Y, Duan SC, Xia QJ, Liang ZC, Dong X, Margaryan K, et al. Dual domestications and origin of traits in grapevine evolution. Science. 2023;379(6635):892–901.
Downey MO, Harvey JS, Robinson SP. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust J Grape Wine R. 2004;10(1):55–73.
Du CL, Cai CL, Lu Y, Li YM, Xie ZS. Identification and expression analysis of invertase family genes during grape Vitis vinifera L. berry development under CPPU and GA treatment. Mol Genet Genomics. 2023;298(3):777–89.
Duan S, Wu Y, Zhang C, Wang L, Song S, Ma C, et al. Differential regulation of enzyme activities and physio-anatomical aspects of calcium nutrition in grapevine. Sci Hortic. 2020;272:109423.
Durán-Soria S, Pott DM, Osorio S, Vallarino JG. Sugar signaling during fruit ripening. Front Plant Sci. 2020;11:564917.
Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW, Qu XQ, et al. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol. 2015;25:53–62.
Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J Exp Bot. 2013;64(6):1451–69.
Farineau J, Lavalmartin D. Light versus dark carbon metabolism in cherry tomato fruits. II. Relationship between malate metabolism and photosynthetic activity. Plant Physiol. 1977;60(6):877–80.
Fontes N, Gerós H, Delrot S. Grape berry vacuole: a complex and heterogeneous membrane system specialized in the accumulation of solutes. Am J Enol Vitic. 2011;62(3):270–8.
Friedel M, Stoll M, Patz C, Will F, Dietrich H. Impact of light exposure on fruit composition of white’Riesling’grape berries (Vitis vinifera L.). Vitis-J Grapevine Res. 2015;54(3):107–16.
Furbank RT, Scofield GN, Hirose T, Wang XD, Patrick JW, Offler CE. Cellular localisation and function of a sucrose transporter OsSUT1 developing rice grains. Aust J Plant Physiol. 2001;28(12):1187–96.
Gambetta GA, Matthews MA, Shaghasi TH, McElrone AJ, Castellarin SD. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta. 2010;232(1):219–34.
Gambetta JM, Holzapfel BP, Stoll M, Friedel M. Sunburn in grapes: a review. Front Plant Sci. 2021;11:604691.
Garrido A, Engel J, Mumm R, Conde A, Cunha A, De Vos RCH. Metabolomics of photosynthetically active tissues in white grapes: effects of light microclimate and stress mitigation strategies. Metabolites. 2021;11(4):205.
Garrido A, Conde A, Serôdio J, De Vos RCH, Cunha A. Fruit photosynthesis: more to know about where, how and why. Plants (Basel). 2023;12(13):2393.
Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Biol. 2001;52:725–49.
Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16:170–80.
Giovannoni J, Nguyen C, Ampofo B, Zhong SL, Fei ZJ. The epigenome and transcriptional dynamics of fruit ripening. Annu Rev Plant Biol. 2017;68:61–84.
Givan CV. Evolving concepts in plant glycolysis: two centuries of progress. Biol Rev. 1999;74(3):277–309.
Gladstones J. Wine, terroir and climate change. South Australia: Wakefield Press; 2011.
Glassop D, Stiller J, Bonnett GD, Grof CPL, Rae A. An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using RNAi suppression. Funct Plant Biol. 2017;44(8):795–808.
Granot D, David-Schwartz R, Kelly G. Hexose kinases and their role in sugar-sensing and plant development. Front Plant Sci. 2013;4:44.
Grappadelli LC, Morandi B, Manfrini L, O’Connell M. Apoplasmic and simplasmic phloem unloading mechanisms: do they co-exist in Angeleno plums under demanding environmental conditions? J Plant Physiol. 2019;237:104–10.
Griesser M, Savoi S, Bondada B, Forneck A, Keller M. Current knowledge of Berry Shrivel in Grapevine: a review considering multiple approaches. J Exp Bot. 2024;75(8):erae001.
Guo WJ, Nagy R, Chen HY, Pfrunder S, Yu YC, Santelia D, et al. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014;164(2):777–89.
Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kuhn C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006;45(2):180–92.
Hanning I, Baumgarten K, Schott K, Heldt HW. Oxaloacetate transport into plant mitochondria. Plant Physiol. 1999;119(3):1025–31.
Harris J, Kriedemann P, Possingham J. Anatomical aspects of grape berry development. Vitis. 1968;7(2):106–19.
Hayes MA, Davies C, Dry IB. Isolation, functional characterization, and expression analysis of grapevine (Vitis vinifera L.) hexose transporters: differential roles in sink and source tissues. J Exp Bot. 2007;58(8):1985–97.
Hendrickson L, Ball M, Wood J, Chow W, Furbank RT. Low temperature effects on photosynthesis and growth of grapevine. Plant Cell Environ. 2004;27(7):795–809.
Ho LH, Klemens PAW, Neuhaus HE, Ko HY, Hsieh SY, Guo WJ. SlSWEET1a is involved in glucose import to young leaves in tomato plants. J Exp Bot. 2019;70(12):3241–54.
Huang L, Wang M, Liu X, Zhao Q, Ma X. Identification, characterization and expression analysis of the sucrose phosphate synthase gene family in Vitis vinifera. J Biobased Mater Bio. 2022;16(4):564–71.
Huber SC, Huber JL. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Phys. 1996;47:431–44.
Hutin M, Sabot F, Ghesquière A, Koebnik R, Szurek B. A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J. 2015;84(4):694–703.
Jackson DI, Lombard PB. Environmental and management practices affecting grape composition and wine quality – a review. Am J Enol Vitic. 1993;44(4):409–30.
Jang JC, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9(1):5–19.
Jiang SR, An PL, Xia CC, Ma WF, Zhao L, Liang TY, et al. Genome-wide identification and expression analysis of the SUT family from three species of sapindaceae revealed their role in the accumulation of sugars in fruits. Plants (Basel). 2024;13(1):95.
Klemens PAW, Patzke K, Deitmer J, Spinner L, Le Hir R, Bellini C, et al. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013;163(3):1338–52.
Kliewer W. The glucose-fructose ratio of Vitis vinifera grapes. Am J Enol Viticult. 1967;18(1):33–41.
Kliewer W. Concentration of tartrates, malates, glucose and fructose in the fruits of the genus Vitis. Am J Enol Viticult. 1967;18(2):87–96.
Kolb CA, Kaser MA, Kopecký J, Zotz G, Riederer M, Pfundel EE. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001;127(3):863–75.
Kryvoruchko IS, Sinharoy S, Torres-Jerez I, Sosso D, Pislariu CI, Guan D, et al. MtSWEET11, a nodule-specific sucrose transporter of Medicago truncatula. Plant Physiol. 2016;171(1):554–65.
Kuhn N, Guan L, Dai ZW, Wu BH, Lauvergeat V, Gomès E, et al. Berry ripening: recently heard through the grapevine. J Exp Bot. 2014;65(16):4543–59.
Lam HM, Xu X, Liu X, Chen W, Yang G, Wong FL, et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet. 2010;42(12):1053–9.
Lecourieux F, Lecourieux D, Vignault C, Delrot S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in Grapevine cells. Plant Physiol. 2010;152(2):1096–106.
Lecourieux F, Kappel C, Lecourieux D, Serrano A, Torres E, Arce-Johnson P, et al. An update on sugar transport and signalling in grapevine. J Exp Bot. 2014;65(3):821–32.
Lee HG, Seo PJ. Transcriptional activation of sugar transport protein 13 mediates biotic and abiotic stress signaling. Plant Signal Behav. 2021;16(8): e1920759.
Li ZM, Palmer WM, Martin AP, Wang RQ, Rainsford F, Jin Y, et al. High invertase activity in tomato reproductive organs correlates with enhanced sucrose import into, and heat tolerance of, young fruit. J Exp Bot. 2012;63(3):1155–66.
Li XY, Guo W, Li JC, Yue PT, Bu HD, Jiang J, et al. Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol. 2020;182(4):2035–46.
Li YM, Forney C, Bondada B, Leng F, Xie ZS. The molecular regulation of carbon sink strength in grapevine (Vitis vinifera L). Front Plant Sci. 2021;11: 606918.
Li DM, Liu BY, Wang ZP, Li XY, Sun SJ, Ma C, et al. Sugar accumulation may be regulated by a transcriptional cascade of ABA-VvGRIP55-VvMYB15-VvSWEET15 in grape berries under root restriction. Plant Sci. 2022;322:1–12.
Liang XG, Gao Z, Fu XX, Chen XM, Shen S, Zhou SL. Coordination of carbon assimilation, allocation, and utilization for systemic improvement of cereal yield. Front Plant Sci. 2023;14:1206829.
Lijavetzky D, Cabezas JA, Ibáñez A, Rodríguez V, Martínez-Zapater JM. High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genomics. 2007;8:1–11.
Lin IW, Sosso D, Chen LQ, Gase K, Kim SG, Kessler D, et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature. 2014;508(7497):546–9.
Liu Z, de Souza TS, Wu H, Holland B, Dunshea FR, Barrow CJ, et al. Development of phenolic-rich functional foods by lactic fermentation of grape marc: a review. Food Rev Int. 2023:1–20.
Lu MZ, Snyder R, Grant J, Tegeder M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant J. 2020;101(1):217–36.
Lu LZ, Yang YZ, Zhong GY, Liang ZC, Cheng LL. Phytochemical composition and content of red-fleshed grape accessions. Horticulturae. 2023;9(5):579.
Lucas WJ, Groover A, Lichtenberger R, Furuta K, Yadav SR, Helariutta Y, et al. The plant vascular system: evolution, development and functions. J Integr Plant Biol. 2013;55(4):294–388.
Ludwig A, Stolz J, Sauer N. Plant sucrose-H+ symporters mediate the transport of vitamin H. Plant J. 2000;24(4):503–9.
Manning K, Davies C, Bowen HC, White PJ. Functional characterization of two ripening-related sucrose transporters from grape berries. Ann Bot. 2001;87(1):125–9.
Maria JME, Susana SM, Diego L, Maria AP, Roque BM. A DIGE-based quantitative proteomic analysis of grape berry flesh development and ripening reveals key events in sugar and organic acid metabolism. J Exp Bot. 2011;62(8):2521–69.
Martínez-Esteso MJ, Sellés-Marchart S, Lijavetzky D, Pedreño MA, Bru-Martínez R. A DIGE-based quantitative proteomic analysis of grape berry flesh development and ripening reveals key events in sugar and organic acid metabolism. J Exp Bot. 2011;62(8):2521–69.
Martínez-Lüscher J, Kurtural SK. Source-Sink manipulations have major implications for grapevine berry and wine flavonoids and aromas that go beyond the changes in berry sugar accumulation. Food Res Int. 2023;169:112826.
Mathan J, Singh A, Ranjan A. Sucrose transport in response to drought and salt stress involves ABA-mediated induction of OsSWEET13 and OsSWEET15 in rice. Physiol Plant. 2021;171(4):620–37.
Meteier E, La Camera S, Goddard ML, Laloue H, Mestre P, Chong J. Overexpression of the VvSWEET4 transporter in grapevine hairy roots increases sugar transport and contents and enhances resistance to Pythium irregulare, a soilborne pathogen. Front Plant Sci. 2019;10:884.
Meyer S, Melzer M, Truernit E, Hümmer C, Besenbeck R, Stadleret R, et al. AtSUC3, a gene encoding a new Arabidopsis sucrose transporter, is expressed in cells adjacent to the vascular tissue and in a carpel cell layer. Plant J. 2000;24(6):869–82.
Moukarzel R, Parker AK, Schelezki OJ, Gregan SM, Jordan B. Bunch microclimate influence amino acids and phenolic profiles of Pinot noir grape berries. Front Plant Sci. 2023;14:1162062.
Myles S, Boyko AR, Owens CL, Brown PJ, Grassi F, Aradhya MK, et al. Genetic structure and domestication history of the grape. PNAS. 2011;108(9):3530–5.
Nguyen-Quoc B, Foyer CH. A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J Exp Bot. 2001;52(358):881–9.
Ni J, Li J, Zhu R, Zhang M, Qi K, Zhang S, et al. Overexpression of sugar transporter gene PbSWEET4 of pear causes sugar reduce and early senescence in leaves. Gene. 2020;743: 144582.
Nie PX, Wang XY, Hu LP, Zhang HY, Zhang JX, Zhang ZX, et al. The predominance of the apoplasmic phloem-unloading pathway is interrupted by a symplasmic pathway during Chinese jujube fruit development. Plant Cell Physiol. 2010;51(6):1007–18.
Nino-Gonzalez M, Novo-Uzal E, Richardson DN, Barros PM, Duque P. More transporters, more substrates: the Arabidopsis major facilitator superfamily revisited. Mol Plant. 2019;12(9):1182–202.
Nonis A, Ruperti B, Pierasco A, Canaguier A, Adam-Blondon A-F, Di Gaspero G, et al. Neutral invertases in grapevine and comparative analysis with Arabidopsis, poplar and rice. Planta. 2008;229:129–42.
Ollat N, Gaudillère JP. Carbon balance in developing grapevine berries. Acta Horticult. 1997;526:345–50.
Ollat N, Diakou-Verdin P, Carde JP, Barrieu F, Gaudillère JP, Moing A. Grape berry development: a review. J Int Sci Vigne Vin. 2002;36(3):109–31.
Palliotti A, Gatti M, Poni S. Early leaf removal to improve vineyard efficiency: gas exchange, source-to-sink balance, and reserve storage responses. Am J Enol Viticult. 2011;62(2):219–28.
Pao SS, Paulsen IT, Saier MH. Major facilitator superfamily. Microbiol Mol Biol R. 1998;62(1):1–34.
Pegler JL, Grof CP, Patrick JW. Sugar loading of crop seeds - a partnership of phloem, plasmodesmal and membrane transport. New Phytol. 2023;239(5):1584–602.
Perotti MF, Posé D, Martín-Pizarro C. Non-climacteric fruit development and ripening regulation: ‘the phytohormones show.’ J Exp Bot. 2023;74(20):6237–53.
Phukan UJ, Jeena GS, Tripathi V, Shukla RK. MaRAP2-4, a waterlogging‐responsive ERF from Mentha, regulates bidirectional sugar transporter at SWEET10 to modulate stress response in Arabidopsis. Plant Biotechnol J. 2018;16(1):221–33.
Pindo M, Vezzulli S, Coppola G, Cartwright DA, Zharkikh A, Velasco R, et al. SNP high-throughput screening in grapevine using the SNPlex™ genotyping system. BMC Plant Biol. 2008;8:1–6.
Radchuk V, Belew ZM, Gündel A, Mayer S, Hilo A, Hensel G, et al. SWEET11b transports both sugar and cytokinin in developing barley grains. Plant Cell. 2023;35(6):2186–207.
Rafique R, Ahmad T, Ahmed M, Khan MA, Wilkerson CJ, Hoogenboom G. Seasonal variability in the effect of temperature on key phenological stages of four table grapes cultivars. Int J Biometeorol. 2023;67(5):745–59.
Reinders A, Sivitz AB, Ward JM. Evolution of plant sucrose uptake transporters. Front Plant Sci. 2012;3:1–12.
Ren Y, Guo SG, Zhang J, He HJ, Sun H, Tian S, et al. A tonoplast sugar transporter underlies a sugar accumulation QTL in watermelon. Plant Physiol. 2018;176(1):836–50.
Ren RH, Yue XF, Li JN, Xie S, Guo SH, Zhang ZW. Coexpression of sucrose synthase and the SWEET transporter, which are associated with sugar hydrolysis and transport, respectively, increases the hexose content in Vitis vinifera L. grape berries. Front Plant Sci. 2020;11:1–15.
Ren Y, Liao SJ, Xu Y. An update on sugar allocation and accumulation in fruits. Plant Physiol. 2023;193(2):888–99.
Reshef N, Karn A, Manns DC, Mansfield AK, Cadle-Davidson L, Reisch B, et al. Stable QTL for malate levels in ripe fruit and their transferability across Vitis species. Hortic Res. 2022;9:uhac009.
Reynolds AG. Viticultural and vineyard management practices and their effects on grape and wine quality. In: Managing wine quality. Canada: Elsevier; 2022. p. 443–539.
Rogiers SY, Coetzee ZA, Walker RR, Deloire A, Tyerman SD. Potassium in the grape (Vitis vinifera L.) berry: transport and function. Front Plant Sci. 2017;8:1629.
Roig-Oliver M, Nadal M, Clemente-Moreno MJ, Bota J, Flexas J. Cell wall components regulate photosynthesis and leaf water relations of Vitis vinifera cv. Grenache acclimated to contrasting environmental conditions. J Plant Physiol. 2020;244: 153084.
Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14(suppl_1):S185–205.
Ronimus RS, Morgan HW. The biochemical properties and phylogenies of phosphofructokinases from extremophiles. Extremophiles. 2001;5(6):357–73.
Ruan YL. Sucrose metabolism. Gateway to diverse carbon use and sugar signaling. Annu Rev Plant Biol. 2014;65:33–67.
Ruan YL, Llewellyn DJ, Furbank RT. The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell. 2001;13(1):47–60.
Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS. Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol Plant. 2010;3(6):942–55.
Ruffner H. Metabolism of tartaric and malic acids in Vitis: a review-part B. Vitis. 1982;21(247–398):259.
Schneider S, Hulpke S, Schulz A, Yaron I, Holl J, Imlau A, et al. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. 2012;14(2):325–36.
Scofield GN, Hirose T, Aoki N, Furbank RT. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J Exp Bot. 2007;58(12):3155–69.
Seo PJ, Park JM, Kang SK, Kim SG, Park CM. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta. 2011;233(1):189–200.
Shahood R, Torregrosa L, Savoi S, Romieu C. First quantitative assessment of growth, sugar accumulation and malate breakdown in a single ripening berry. Oeno One. 2020;54(4):1077–92.
Sivitz AB, Reinders A, Johnson ME, Krentz AD, Grof CPL, Perroux JM, et al. Arabidopsis sucrose transporter AtSUC9. High-affinity transport activity, intragenic control of expression, and early flowering mutant phenotype. Plant Physiol. 2007;143(1):188–98.
Slewinski TL. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: a physiological perspective. Mol Plant. 2011;4(4):641–62.
Slewinski TL, Meeley R, Braun DM. Sucrose transporter1 functions in phloem loading in maize leaves. J Exp Bot. 2009;60(3):881–92.
Slewinski TL, Garg A, Johal GS, Braun DM. Maize SUT1 functions in phloem loading. Plant Signal Behav. 2010;5(6):687–90.
Smart RE. Principles of grapevine canopy microclimate manipulation with implications for yield and quality. A review. Am J Enol Viticult. 1985;36(3):230–9.
Smeekens S. Sugar-induced signal transduction in plants. Annu Rev Plant Biol. 2000;51(1):49–81.
Somkuwar RG, Sharma AK, Oulkar DP. Bunch exposure of syrah vines affect bunch and berry quality. Environ Ecol Stat. 2023;41:1149–56.
Stanfield RC, Forrestel EJ, Elmendorf KE, Bagshaw SB, Bartlett MK. Phloem anatomy predicts berry sugar accumulation across 13 wine-grape cultivars. Front Plant Sci. 2024;15: 1360381.
Sturm A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999;121(1):1–8.
Sun WJ, Gao ZY, Wang J, Huang YQ, Chen Y, Li J, et al. Cotton fiber elongation requires the transcription factor GhMYB212 to regulate sucrose transportation into expanding fibers. New Phytol. 2019;222(2):864–81.
Sweetman C, Deluc LG, Cramer GR, Ford CM, Soole KL. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry. 2009;70(11–12):1329–44.
Taureillessaurel C, Romieu CG, Robin JP, Flanzy C. Characterization of the major mitochondrial and cytosolic isoforms and their role in ripening. Am J Enol Vitic. 1995;46(1):29–36.
Tian L, Jia HF, Li CL, Fan PG, Xing Y, Shen YY. Sucrose accumulation during grape berry and strawberry fruit ripening is controlled predominantly by sucrose synthase activity. J Hortic Sci Biotech. 2012;87(6):661–7.
Venios X, Korkas E, Nisiotou A, Banilas G. Grapevine responses to heat stress and global warming. Plants (Basel). 2020;9(12):1754.
Verma AK, Upadhyay S, Verma PC, Solomon S, Singh SB. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. 2011;13(2):325–32.
Vignault C, Vachaud M, Cakir B, Glissant D, Dédaldéchamp F, Büttner M, et al. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot. 2005;56(415):1409–18.
Viola R, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, et al. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. Plant Cell. 2001;13(2):385–98.
Wan H, Wu L, Yang Y, Zhou G, Ruan YL. Evolution of sucrose metabolism: the dichotomy of invertases and beyond. Trends Plant Sci. 2018;23(2):163–77.
Wang X, Li L, Yang P, Gong C. The role of hexokinases from grape berries (Vitis vinifera L.) in regulating the expression of cell wall invertase and sucrose synthase genes. Plant Cell Rep. 2014;33:337–47.
Wang L, Lu QT, Wen XG, Lu CM. Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol. 2015;169(4):2848–62.
Wang GP, Wu Y, Ma L, Lin Y, Hu YX, Li MZ, et al. Phloem loading in rice leaves depends strongly on the apoplastic pathway. J Exp Bot. 2021;72(10):3723–38.
Wen SY, Neuhaus HE, Cheng JT, Bie ZL. Contributions of sugar transporters to crop yield and fruit quality. J Exp Bot. 2022;73(8):2275–89.
Xia Q, Guo Y, Zhang Z, Li D, Xuan Z, Li Z, et al. Complete resequencing of 40 genomes reveals domestication events and genes in silkworm (Bombyx). Science. 2009;326(5951):433–6.
Xia H, Shen Y, Deng H, Wang J, Lin L, Deng Q, et al. Melatonin application improves berry coloration, sucrose synthesis, and nutrient absorption in ‘Summer Black’ grape. Food Chem. 2021;356:129713.
Xin H, Zhang J, Zhu W, Wang N, Fang P, Han Y, et al. The effects of artificial selection on sugar metabolism and transporter genes in grape. Tree Genet Genomes. 2013;9:1343–9.
Xu QY, Yin SJ, Ma Y, Song M, Song YJ, Mu SC, et al. Carbon export from leaves is controlled via ubiquitination and phosphorylation of sucrose transporter SUC2. PNAS. 2020;117(11):6223–30.
Xue X, Wang J, Shukla D, Cheung LS, Chen LQ. When SWEETs turn tweens: updates and perspectives. Annu Rev Plant Biol. 2022;73:379–403.
Yang WW, Zhu JQ, van Leeuwen C, Dai ZW, Gambetta GA. GrapevineXL reliably predicts multi-annual dynamics of vine water status, berry growth, and sugar accumulation in vineyards. Hortic Res. 2023;10(6):uhad071.
Yuan M, Chu ZH, Li XH, Xu CG, Wang SP. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell. 2010;22(9):3164–76.
Zeng L, Wang Z, Vainstein A, Chen S, Ma H. Cloning, localization, and expression analysis of a new tonoplast monosaccharide transporter from Vitis vinifera L. J Plant Growth Regul. 2011;30:199–212.
Zeng Z, Lyu T, Lyu YM. LoSWEET14, a sugar transporter in Lily, is regulated by transcription factor LoABF2 to participate in the ABA signaling pathway and enhance tolerance to multiple abiotic stresses in tobacco. Int J Mol Sci. 2022;23(23):1–19.
Zenoni S, Savoi S, Busatto N, Tornielli GB, Costa F. Molecular regulation of apple and grape ripening: exploring common and distinct transcriptional aspects of representative climacteric and non-climacteric fruits. J Exp Bot. 2023;74(20):6207–23.
Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, et al. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiol. 2006;142(1):220–32.
Zhang Q, Hu WC, Zhu F, Wang LM, Yu QY, Ming R, et al. Structure, phylogeny, allelic haplotypes and expression of sucrose transporter gene families in Saccharum. BMC Genomics. 2016;17(1):1–18.
Zhang Z, Zou LM, Ren C, Ren FR, Wang Y, Fan PG, et al. VvSWEET10 mediates sugar accumulation in grapes. Genes (Basel). 2019;10(4):1–18.
Zhang Y, Chang BM, Burdet B, Dai ZW, Delrot S, Keller M. Apoplastic sugar may be lost from grape berries and retrieved in pedicels. Plant Physiol. 2022;190(1):592–604.
Zhang SH, Wang H, Wang T, Zhang J, Liu WJ, Fang HC, et al. Abscisic acid and regulation of the sugar transporter gene MdSWEET9b promote apple sugar accumulation. Plant Physiol. 2023;192(3):2081–101.
Zhong H, Yadav V, Wen Z, Zhou X, Wang M, Han S, et al. Comprehensive metabolomics-based analysis of sugar composition and content in berries of 18 grape varieties. Front Plant Sci. 2023;14: 1200071.
Zhou YX, Li KX, Wen SY, Yang D, Gao J, Wang ZW, et al. Phloem unloading in cultivated melon fruits follows an apoplasmic pathway during enlargement and ripening. Hortic Res. 2023;10(8):uhad123.
Zhu F, Wen W, Cheng Y, Fernie AR. The metabolic changes that effect fruit quality during tomato fruit ripening. Mol Hortic. 2022;2(1):1–19.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Key Research and Development Program of China (2022YFE0116400), the National Natural Science Foundation of China (32025032), CAS Project for Young Scientists in Basic Research (YSBR-093), and the joint laboratory Innogrape (IBCAS, INRAE, University of Bordeaux, Bordeaux Sciences Agro).
Author information
Authors and Affiliations
Contributions
Z.L. and L.L designed the outline of this manuscript. L.L wrote the Abstract, Introduction and the main text of manuscript. S.D. and Z.L. made extensive revision and generated the final version. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Prof. Zhenchang Liang is a member of the Editorial Board for Molecular Horticulture. He was not involved in the journal’s review of, and decisions related to, this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, L., Delrot, S. & Liang, Z. From acidity to sweetness: a comprehensive review of carbon accumulation in grape berries. Mol Horticulture 4, 22 (2024). https://doi.org/10.1186/s43897-024-00100-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43897-024-00100-8