Abstract
The increasing number of approved nucleic acid therapeutics demonstrates the potential for the prevention and treatment of a broad spectrum of diseases. This trend underscores the significant impact and promise of nucleic acid-based treatments in the field of medicine. Nevertheless, employing nucleic acids as therapeutics is challenging due to their susceptibility to degradation by nucleases and their unfavorable physicochemical characteristics that hinder delivery into cells. Appropriate vectors play a pivotal role in improving nucleic acid stability and delivering nucleic acids into specific cells. The maturation of delivery systems has led to breakthroughs in the development of therapeutics based on nucleic acids such as DNA, siRNA, and mRNA. Non-viral vectors have gained prominence among the myriad of nanomaterials due to low immunogenicity, ease of manufacturing, and simplicity of cost-effective, large-scale production. Here, we provide an overview of the recent advancements in nanomaterials for nucleic acid delivery. Specifically, we give a detailed introduction to the characteristics of polymers, lipids, and polymer-lipid hybrids, and provide comprehensive descriptions of their applications in nucleic acid delivery. Also, biological barriers, administration routes, and strategies for organ-selective delivery of nucleic acids are discussed. In summary, this review offers insights into the rational design of next-generation delivery vectors for nucleic acid delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
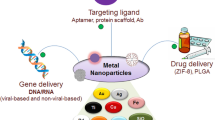
Nucleic acid therapy offers a novel therapeutic modality for congenital and acquired diseases by delivering exogenous nucleic acids into lesions to modulate the expression of proteins [1,2,3,4,5] (Fig. 1). Compared to conventional small-molecule and antibody drugs, nucleic acid drugs have advantages of short development cycle, abundant choice of targets and remarkable curative effect [6,7,8,9,10]. Furthermore, nucleic acid drugs offer a solution to circumvent the limitations associated with undruggable targets [11,12,13]. These advancements provide potential treatment alternatives for previously considered incurable conditions [6, 14]. With the rapid development of molecular biology techniques such as in vitro transcription (IVT) and Clustered regularly interspaced short palindromic repeats associated (CRISPR-Cas) gene editing, nucleic acid drugs have made tremendous advances in the prevention and treatment of a variety of diseases [15,16,17,18,19].
Schematic illustration depicting nanoparticles' route and functional mechanisms within cells. When nanoparticles contact cells, they are recognized and internalized by the cell membrane. Inside the cell, the lysosome-endosome system engulfs the nanoparticles, where the cargo they carry is subsequently released. a mRNA is translated by the ribosome in the cytoplasm, and the resulting protein may be processed by the golgiosome for secretion or presented as an antigen. b siRNA binds with the RNA-Induced Silencing Complex (RISC) in the cytoplasm, inhibiting the translation of mRNA transcribed from genes. c pDNA crosses the nuclear membrane and undergoes transcription in the cell nucleus. Created with BioRender.com
Naked nucleic acids face numerous challenges in in vivo delivery [20]. The key to achieving the complete functions of nucleic acid drugs is the choice of delivery vectors [21]. Ideally, these vectors should protect nucleic acids from enzymatic degradation, facilitate cellular uptake and endosomal escape, and exhibit minimal toxicity. Viral vectors, such as adenovirus, lentivirus, and adeno-associated virus, have been developed to enable efficient cellular uptake [22,23,24]. Several nucleic acid drugs delivered using viral vector platforms, such as Luxturna® and Zolgensma®, have received approval from the U.S. Food and Drug Administration (FDA) [25]. Generally, viral vector-based nucleic acid drugs have long-lasting efficacy over conventional medications, only a single injection of these nucleic acid drugs maintains therapeutic efficacy for a considerable length of time [22, 26,27,28,29]. Nevertheless, viral vectors can potentially induce antiviral immune responses, which poses a challenge for repeat administration [26, 29, 30]. In addition, adverse reactions of abnormal blood clots occurred in some instances of clinical use of viral vector nucleic acid drugs [29,30,31].
There has been a growing interest in structuring non-viral vectors in recent years. Compared with viral vectors, non-viral vectors exhibit low immunogenicity without the risk of insertional mutagenesis and ease of large-scale production [32, 33]. Conventional non-viral vectors generally suffer from low transfection efficiency and limited tissue targeting [34]. Fortunately, novel nanomaterials with superior cell transfection and active tissue targeting capability have emerged [35, 36]. For instance, lipid nanoparticles (LNPs) have shown significant potential in nucleic acid drug delivery, as evidenced by numerous preclinical and clinical studies [37, 38]. One notable example is the siRNA-LNP drug Onpattro® (patisiran), which has gained positive outcomes in treating Hereditary Transthyretin-Mediated (hATTR) amyloidosis with only one dose every three weeks and received FDA’s approval in 2018 [39,40,41,42]. Since Onpattro's approval, five siRNA-based drugs have been available on the market, and over 200 siRNA therapies are currently in development.
Most recently, the global SARS-CoV-2 pandemic has triggered an unprecedented emergence of mRNA-based vaccines for infectious diseases. Among these candidates, BNT162b2 (Comirnaty®) by BioNTech/Pfizer and mRNA-1273 (Spikevax®) by Moderna have demonstrated high effectiveness in the prevention and control of SARS-CoV-2 [43, 44]. mRNA vaccines have great promise in terms of preventing and treating pandemics owing to high efficacy, ease of manufacture, scalable production, and high success rate of clinical trials [45,46,47]. In March 2023, SYS6006, an mRNA vaccine developed by the CSPC Pharmaceuticals Group, became China's first domestic COVID-19 mRNA vaccine for emergency use. A recent survey identified 966 vaccine candidates in development worldwide, of which approximately 20% are nucleic acid-based vaccines [48]. They have the potential to usher in a new era of pandemic prevention and control, opening up new opportunities for drug discovery and development [46, 49,50,51,52,53]. So far, nucleic acid drugs have remarkably progressed in treating liver, eye, and cardiovascular diseases [54,55,56,57,58,59,60].
In this review, we focus on recent advances in nanomaterials, including polymers, lipids, and polymer-lipid hybrids, as vehicles for nucleic acid delivery. The physiological barriers and the diverse administration routes are also described. Understanding these factors is crucial for rationally designing optimal vectors for targeted delivery of nucleic acids to ensure both efficacy and safety. Overall, we aim to offer insights into developing next-generation delivery carriers for nucleic acids with optimal properties.
Biological barriers for nucleic acid delivery
Systemic barriers
Before reaching target cells, nanoparticles face a barrier known as the extracellular barrier, which exists within the bloodstream and intercellular matrix (Fig. 2a). This barrier primarily includes the complex components in the blood. The blood serum contains a large number of nucleases, including endonucleases and exonucleases, which can hydrolyze the phosphodiester bonds of exposed nucleic acids and inactivate them [61]. In early research concerning nucleic acid delivery, nucleic acids were tightly bound to carrier materials or chemically modified to prevent nuclease-induced degradation. However, these strategies have several drawbacks compared to the utilization of advanced delivery carriers [62]. Like all foreign substances, once nanoparticles are recognized by the mononuclear phagocyte system (MPS), they often undergo degradation and trigger undesired immune reactions in inappropriate sites. After entering the bloodstream, the surface of nanoparticles quickly adsorbs a layer of proteins, including opsonins, serum albumin, complement, and others, referred to as the “protein corona” [63,64,65]. The presence of the protein corona not only enhances the phagocytosis of nanoparticles by phagocytic cells [65] but may also mask ligands on the nanoparticle surface responsible for active targeting [66], leading to a decrease in nanoparticle specificity. It has been found that polyethylene glycol (PEG) modification prevents protein corona formation and reduces MPS clearance [67]. Similarly, Tasciotti et al. developed a coating of bionic particles consisting of cell membranes isolated from leukocytes, reducing MPS's conditioning effect [68].
Organ barriers
Nanoparticles targeting organs other than the liver and kidneys also face the risk of hepatic and renal clearance, mainly related to the nanoparticles' properties (size, surface charge, etc.). Nanoparticles with a diameter of less than 5 nm are rapidly excreted through the kidneys after entering the bloodstream. In contrast, larger nanoparticles are eliminated by the liver's reticuloendothelial system (RES) [69, 70]. In addition, particle size is related to the passage of nanoparticles to tissue-specific structures. For example, nanoparticles targeting the spleen must also overcome the splenic barrier after spilling out of the blood vessels. Macrophages in the red medulla and marginal zone of the spleen prevent the nanoparticles from entering the white medulla, thus lessening the strength of the immune response [69]. Only nanoparticles with appropriate size can cross the marginal zone through the bridging channel and enter the T-cell and B-cell zones in the white medulla, which are densely populated with lymphocytes that can quickly and efficiently initiate an adaptive immune response against specific antigens. Moreover, the human body houses various organs, such as the brain and placenta, which possess robust protective mechanisms. Additional obstacles must be overcome to successfully transport nanoparticles to these organs, such as the blood–brain barrier for delivering nucleic acids to the brain and the blood-labyrinth barrier for targeting the inner ear [71,72,73].
The case of the lungs is even more specific, where the delivery of nanoparticles to the lungs via the respiratory tract, such as inhalation, must overcome a complex respiratory barrier (Fig. 2b). The mucus-cilia clearance system (MC) on the airway epithelium is an initial barrier to overcome [74]. Airway mucus, derived mainly from submucosal glands and goblet cells, consists of mucin fibers forming a dense meshwork [75]. The mucus layer immobilizes inhaled foreign bodies, including nucleic acid-nanocarrier complexes, through electrostatic interactions and removes them from binding sites. Studies have shown that only particles smaller than 40 nm can effectively penetrate the mucus layer through passive Brownian motion [76]. In diseases like cystic fibrosis and asthma, excessive mucus accumulation hinders drug delivery and induces coughing, which further clears foreign substances [77]. Researchers are attempting to overcome the barriers of mucociliary clearance by utilizing material properties [78, 79]. Angelo and others have developed mucus-inert nanomaterials that exploit the intrinsic properties of the mucus layer to enable sustained siRNA release within the mucus layer [80]. Additionally, due to proteins, lipids, and ions in the airways, cationic polymer- and lipid-based carriers interact readily with negatively charged mucus components and aggregate [81]. Surfactant, a lipoprotein secreted by alveolar type II epithelial cells, is another barrier to the pulmonary delivery of nucleic acids. It comprises dipalmitoyl phosphatidylcholine (DPPC) and surfactant-binding protein (SP) and significantly affects the bio-efficacy of RNA-nanoparticle complexes [82]. In animal experiments, adding the surfactant “Alveofact” reduced the transfer efficiency of DNA-PEI complexes [83].
Cellular barriers
Once nanoparticles successfully circumvent the barriers mentioned above and approach target cells, the cell membrane becomes the first obstacle encountered during cellular uptake. Unlike small molecule drugs, nanoparticles rely on active uptake mechanisms to enter cells, including receptor-mediated endocytosis, macropinocytosis, and caveolae-mediated endocytosis [84]. Overall, cellular uptake of nanoparticles can be categorized into two types: phagocytosis and pinocytosis [84, 85]. Phagocytosis only occurs in phagocytic cells such as monocytes, neutrophils, and macrophages, while pinocytosis is widely present in various cell types. The cellular uptake of nanoparticles is typically receptor-mediated, where ligands on the nanoparticles bind to specific receptors on the cell membrane, leading to membrane invagination. Different nanoparticles employ distinct pathways to breach the cell membrane. Kubota et al. treated HeLa cells with specific inhibitors targeting different uptake pathways and found that cellular uptake of lipid nanoparticles (LNPs) mainly relies on caveolae-mediated endocytosis, while the uptake of lipoplexes depends on at least two pathways [86]. Efficient cellular uptake is a prerequisite for enhancing the cell availability of nanocomplexes carrying nucleic acids. The composition, size, surface charge, and shape of nanoparticles can influence cellular uptake efficiency [86,87,88]. Notably, the protein corona is not the sole factor affecting the interaction between nanoparticles and target cells. Suberi et al. found that the content of PEG on the surface of polymers influences their cellular uptake efficiency. However, this influence is not linear. In-depth studies have revealed that high PEG density can affect the peripheral conformation of polymers, impacting the interaction between nucleic acid-loaded nanoparticles and target cells [89]. Hatakeyama et al. also reached a similar conclusion when using a different material to deliver siRNA [90].
The vesicles formed during the endocytosis mentioned above fuse with early endosomes within the cell and gradually mature into late endosomes through acidification, ultimately combining with lysosomes. Lysosomes are acidic organelles within the cell with a firm acidity (pH around 5 [91, 92]) and contain various enzymes. Therefore, if the nanocarriers loaded with nucleic acids cannot escape promptly, both the carrier and its cargo will be destroyed in the extreme environment of the lysosome. Different materials used to form nanoparticles have different mechanisms for endosomal escape. However, most nanoparticles, including cationic lipid nanoparticles, disrupt the negatively charged endosomal membrane through electrostatic interactions [93, 94]. For example, it is widely believed that some cationic polymers interact with the endosomal membrane through the “proton sponge effect” and subsequently disrupt it (which will be described in detail in the corresponding section) [95]. This interaction can also be achieved using ionizable lipids, as these pH-sensitive lipids can be protonated in acidic environments, acquiring a positive charge [96]. It is worth noting that some helper lipids can also be protonated in acidic environments. For example, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) with a hexagonal inverted cone structure can form ion pairs with membrane phospholipids in the endosome, thereby promoting the process of endosomal escape [97]. Another promising approach is to conjugate viral-derived proteins or peptides on the surface of nanoparticles, utilizing the natural entry mechanisms of viruses to achieve endosomal escape. For example, the HIV-1 transmembrane protein gp41 and H5WYG are derived from the influenza virus [98,99,100].
Delivery routes for nucleic acids
Systemic routes
Systemic administration mainly includes subcutaneous injection (SC), intramuscular injection (IM), intradermal injection (ID), and intravenous injection (IV) (Fig. 3). Among them, the nanoparticles administered via SC, IM, and ID are not directly entering the bloodstream but are taken up by APCs in the subcutaneous or muscle tissue [101]. Each of these injection methods has its pros and cons. For example, it is generally believed that subcutaneous injection has a lower capillary density, allowing the antigen to be continuously released at a certain rate after SC [102]. Still, some argue that SC is an outdated immunization method [103]. Although nucleic acid drugs or vaccines administered through SC, IM, and ID mainly activate immune cells in peripheral lymph nodes, it is difficult to achieve targeted delivery to central immune organs or other specific tissues to date. Owing to their higher safety and patient compliance compared to IV, these routes remain the mainstay of preventive vaccination. Generally, most nanoparticles targeting a specific tissue efficiently rely on the IV route. In this case, nanoparticles enter the bloodstream at the fastest rate after injection and participate in blood circulation. Nucleic acid-nanocarrier complexes tend to passively target tissues with abundant blood supply, especially for nucleic acid drugs targeting the liver, IV is an excellent delivery route.
Despite the advancements in nucleic acid delivery technologies using various materials, achieving efficient delivery of nucleic acid-nanocarrier complexes via systemic administration to extrahepatic tissues remains challenging [104,105,106]. When nucleic acid-nanocarrier complexes are injected intravenously, due to the dependence on the circulatory system for drug transport from the injection site to the target cells, a large amount of the complex is intercepted mainly by hepatocytes, resulting in a significant release of nucleic acids in the liver, and consequently, a lower-than-anticipated concentration of the drug reaching the target tissues. Researchers have thought of many ways to achieve extrahepatic targeting of nucleic acids. In the case of LNPs-RNA, for example, the scientific community has been exploring three main directions to achieve extrahepatic targeting. These approaches aim to enhance the targeted delivery of nucleic acid-nanocarrier complexes to the target tissues and improve their therapeutic efficacy while overcoming the challenges associated with systemic administration.
-
1).
Pre-injecting a large number of irrelevant liposomes (Nanoprimers) to occupy hepatocyte sites and slightly weaken the uptake of LNPs by hepatocytes [107]. Regardless, the clinical translational potential of this approach is limited.
-
2).
Modifying ligands by splicing to improve the existing LNPs. For example, Lee et al. enhanced the delivery and transfection efficiency of siRNA in cancer cells by conjugating the EphA2 ligand, Ephrin-A1, on the surface of LNPs, taking advantage of the overexpression of the EphA2 receptor in most cancer cells [108].
-
3).
Designing and synthesizing new LNPs with endogenous targeting functions. Qiu et al. discovered that LNPs containing an amide bond in their lipid tails selectively delivered mRNA to mouse lung cells, especially alveolar endothelial cells. They also altered the selectivity of drug delivery to alveolar endothelial cells or alveolar macrophages by adjusting the structure of the head of lipids [109]. Moreover, changing the components of LNPs can achieve selective targeting of tissues and organs.
Respiratory routes
Given the challenges of systemic drug delivery, topical delivery techniques have received increasing attention in recent years [110]. Theoretically, the respiratory route is optimal for lung epithelial cells targeting [111]. This approach has successfully prevented and treated viral infections and respiratory diseases [112,113,114,115]. Unlike other organs, the lungs are directly exposed to the external environment through the respiratory tract, and the mucosa of the respiratory system provides a vast absorptive surface area. This unique characteristic makes the delivery of nucleic acid-nanocarrier complexes to the lungs through intratracheal/intranasal drops and nebulization a highly promising strategy [116,117,118]. By delivering the complexes via the respiratory tract, complexes can directly reach cells, such as alveolar epithelial cells, in the pulmonary airways and alveoli without relying on systemic circulation. This approach achieves efficient drug delivery and minimizes drug loss [119].
Inhalation presents great potential among various routes for delivering nucleic acid-nanocarrier complexes to the respiratory tract [120]. This route typically requires the use of specialized devices. Early equipment involved metered-dose inhalers (MDIs), which deliver drugs dissolved or suspended in a liquid propellant (e.g., hydrofluoroalkanes) to achieve rapid and convenient drug delivery. Later, dry powder inhalers (DPIs) were introduced to overcome the problem of limited drug deposition in the lower respiratory tract by MDIs, which deliver drugs in the form of solid aerosols. Nevertheless, the efficiency of dry powder inhalers is mainly dependent on the patient's respiratory function, posing new challenges for quality control stability [121]. Subsequently, nebulization devices were developed. Dolovich et al. demonstrated that the liquid aerosols produced by nebulizers, capable of carrying hundreds of nanoparticles per drop, can reach nearly all regions of the lungs [122]. Philip J. Santangelo [123, 124] and Daniel G. Anderson's team [114] achieved effective deposition and efficient transfection of functional mRNA in the lungs of experimental animals using devices such as small animal vibrating sieve mesh nebulizers and nebulizing towers, demonstrating the role of nebulization in delivering nucleic acids to counter respiratory pathogens. It's worth mentioning that ALN-RSV01, a nebulized siRNA therapy targeting the RSV nucleocapsid protein (N), has been clinically validated and proven safe and effective in over 3,000 symptomatic patients [125]. Despite that, nebulized drug delivery also faces many challenges due to the instability of liquid nucleic acid-nanocarrier complexes. Nebulization devices like jet and ultrasonic nebulizers may apply a continuous shear force to the complexes, leading to nanoparticle coalescence. Consequently, nebulization technology poses challenges related to delivery materials, buffer systems, and nebulization device design [126].
Organ-selective nucleic acid delivery
Due to the unique physiological configuration and function of the liver, nucleic acids carried by nanomaterials such as LNPs tend to aggregate in the liver [127], granting nucleic acids unique advantages in treating liver diseases. So far, among the five siRNA drugs approved by the FDA, except for Onpattro®, all have employed GalNAc-based modifications. This proven strategy allows nucleic acid drugs to achieve efficient liver parenchymal cell targeting [128, 129]. However, the liver has a complex cellular composition. In addition to hepatic parenchymal cells, the hepatic microenvironment includes hepatic stellate cells (HSCs), Kupffer cells (hepatic macrophages), and hepatic endothelial cells [130], which have been associated with a wide range of liver diseases and metabolic disorders. For example, HSCs are considered significant cells in the formation of hepatic fibrosis and are closely associated with various chronic liver diseases [131]. It is necessary to develop better strategies targeting multiple cell types.
Efficiently modulating the immune system is crucial for treating diseases such as infectious diseases, tumors, and autoimmune diseases. In this regard, nucleic acid drugs also have advantages. The liver is also a component of the body's immune system [132]. However, secondary lymphoid organs, including lymph nodes (LN) and spleen, are the core foundation of immune responses [133]. The spleen, the largest lymphoid organ, stores about one-third of circulating lymphocytes and possesses a large number of antigen-presenting cells (APCs) and tissue-resident lymphocytes [134]. After intravenous administration, nanocomplexes targeting the spleen can be internalized by the large number of APCs in the spleen, resulting in mighty and enduring cellular and humoral immunity [135]. Thus, the spleen has become another promising organ for nucleic acid delivery, which has greatly attracted a lot of interest.
Unlike other organs, the lungs are immediately exposed to the external environment through the respiratory tract, making them susceptible to a wide range of pathogens [136]. Recently, increasing environmental pollution and outbreaks of pandemics, such as Influenza, respiratory syncytial virus (RSV) infection and COVID-19 [137, 138], have led to a significant rise in the incidence of pulmonary diseases. It has imposed an enormous burden on society and even triggered global public health crises on multiple occasions [139, 140]. Nucleic acid drugs present remarkable strengths in the treatment of inherited lung diseases [141], lung cancer [142], asthma [143], and infectious pneumonia [144]. Over the past few years, several lung-targeted nucleic acid drugs, including MRT5005®, RCT1100®, VX-522®, and ARO-ENaC®, have entered clinical trials (Table 1). However, the majority of lung-targeted nucleic acid drugs remain in the preclinical stage, restricting breakthroughs in these studies due to the lack of appropriate delivery materials [145,146,147,148].
Nanomaterials for nucleic acid delivery
To protect nucleic acids and ensure efficient transfection, encapsulating them into nanoparticles using safe materials is often necessary. However, selecting suitable materials presents a challenge, as they must achieve effective encapsulation, be taken up by target cells, and successfully deliver the gene to the intended site of action within the cell. Additionally, these materials should possess good biosafety and degradability properties. The success of organ-targeted nucleic acid delivery using nanomaterials should circumvent various extracellular barriers and intracellular barriers. Different routes of drug delivery are associated with distinct physiological barriers. In the case of respiratory tract delivery, the challenge lies in overcoming the barriers presented by the highly branched respiratory tract, characterized by varying diameters and lengths. Nanoparticles, due to their nanoscale dimensions, are quickly exhaled. Therefore, specific methods, such as integrating nanoparticles with excipients, need to be employed to confer inhalation characteristics upon the particles. Furthermore, respirable particles tend to accumulate in the mucus layer, preventing their entry into cells. When administered via intravenous injection, drugs must traverse the vessel wall and be endocytosed by cells, subsequently releasing the encapsulated nucleic acids through endosomes. Consequently, delivery carriers for nucleic acids should possess enhanced endosomal escape capabilities. A comprehensive understanding of diverse nanomaterials' chemical properties and applications is crucial in the rational development of more effective and selective nucleic acid delivery vehicles catering to treating specific tissues and cells.
Polymer-based delivery system
Polyethyleneimine (PEI)
Polyethylenimine (PEI) is a highly charged cationic polymer that easily binds negatively charged nucleic acids to form complexes transfected into adherent and suspension cells, and is commonly used for transient gene transfer (Fig. 4a). Moreover, PEI is easily synthesized, allowing for flexible adjustment of its physicochemical properties. It demonstrates significant transfection efficiency in delivering nucleic acids in vivo. The main advantage of PEI as a nucleic acid delivery material lies in its efficient endosomal escape. The “proton sponge effect” is considered one of the critical principles. PEI can capture a large number of protons. Then, the influx of chloride ions and water into endosomes/lysosomes disrupts osmotic pressure homeostasis, leading to endosomal rupture and subsequent release of nucleic acids [95]. These characteristics have positioned PEI as extensively employed vectors for the delivery of various nucleic acids, including plasmid DNA and siRNA, to diverse tissues [149,150,151]. However, this disruptive effect is not limited to endosomal membranes, the uncontrollable proton sponge effect along with the high-density charge of the polymer, can potentially damage cell membranes, and mitochondria and even induce cell necrosis [152]. Moreover, the difficulty of PEI biodegradation and its cytotoxicity have limited its widespread application as a nucleic acid delivery material [153].
a Schematic illustration, chemical structures, and comparison of advantages and drawbacks of several polymers used as nucleic acids nanocarriers. Created with BioRender.com. b hDD90-118 was synthesized by adding N-methyl-1,3-diaminopropane, a tri-functional amine. Bioluminescence 24 h after inhalation of hDD90-118 polyplexes. hDD90-118 vectors produced significantly higher radiance localized to the lung. A Cre-loxP mouse model was utilized to quantitatively assess the lung cell subtypes transfected by hDD90-118 polyplexes. The tdTomato + lung cells were typed using flow cytometry, where endothelial cells were labeled with CD31, epithelial cells with EpCAM, and immune cells with CD45. Reprinted with permission [114]. Copyright 2019, Wiley. c The schematic of the PBAE used in the article. IVIS shows the biodistribution of luciferase expression in dissected mouse organs at a nanoparticle-to-DNA ratio of 50:1. IVIS whole-body imaging shows the in vivo bioluminescence following treatment with PBAE nanoparticles. Flow cytometry analysis of the PBAE nanoparticle distribution (blue curve) in different respiratory cell types compared to the control (red curve). Flow cytometry shows mCherry reporter activity in endothelial cells isolated from different murine organs. PBAE nanoparticles (blue curve); Untreated control (red curve). Immunofluorescence of frozen lung sections after I.V. injection of DyLight 650-labeled nanoparticles. The PBAE nanoparticles (light blue); Endothelial cells (CD31, red); Smooth muscle cells (αSMA, green); nucleus (DAPI, dark blue). Reprinted with permission [154]. Copyright 2023, KeAi
To address these limitations, efforts are being made to improve the safety of PEI while maintaining its delivery efficiency. Strategies such as reducing the molecular weight of PEI, decreasing branching structures (e.g., deacetylation), hydrophobic modification, or conjugation with other polymers (e.g., PEG, chitosan, hyaluronic acid) to form nanoparticles have shown promise [155, 156]. For example, using PEG-PEI as a siRNA delivery system may reduce cytotoxicity while maintaining the target gene silencing effect. It should be noted that PEGylated modifications have the potential to initiate immune responses and inflammatory responses in the lungs but are generally not sufficient to cause lung tissue damage [126, 157]. In another example, it was observed that the 32P-siRNA-PEI complex showed a minimal reduction in the radiolucent signal detected after treatment compared to the naked 32P-siRNA. Of note, the presence of PEI considerably influenced the uptake of 32P-siRNA by bronchoalveolar lavage cells (BAL cells) [158, 159].
Daniel J. Siegwart’s group found that fluorinated PEI successfully mediated siRNA binding in the triple-negative breast cancer (TNBC) cell line, MDA-MB-231, while reducing cytotoxicity and aggregation compared to unmodified PEI. Moreover, fluorinated PEI caused a distinct shift in the biodistribution of siRNA from the lungs to the liver [103]. Fernando et al. showed that pDNA was transfected into retinal pigment epithelium (ARPE-19) and human hepatocellular carcinoma (HepG2) cell lines via succinic acid-modified PEI. They found that adding succinic acid slightly reduced the strength of the polymer-DNA interaction, resulting in better intracellular DNA release and reduced cytotoxicity by avoiding the adsorption of proteins onto the polymers [160]. In a separate study, Kurosaki et al. observed that the positive charge of the pDNA/PEI complex was effectively shielded by the addition of 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS). In their finding, the gene was selectively highly expressed in the spleen after intravenous injection of the pDNA/PEI/DOPS ternary complex [161]. Yang et al. designed a biocompatible biomimetic system based on nano-erythrocyte bodies (NER) and black phosphorus nanosheets (BP) to achieve spleen targeting. BP was covalently modified with PEI and served as the core to efficiently condense mRNA via electrostatic interactions, forming NER@BPmRNA. In in vivo experiments, they demonstrated that NER@BP, when injected into the muscle, effectively targeted the spleen for antigen delivery [162].
Chitosan
Chitosan is a naturally occurring and biocompatible polysaccharide material that exhibits positive electrical properties due to the presence of amine groups [163,164,165] (Fig. 4a). These positive charges enable chitosan to encapsulate nucleic acids effectively. In addition, chitosan possesses immunomodulatory properties and, therefore, has the potential to be used as an adjuvant for nucleic acid vaccines [166, 167]. The adhesive and mucosal permeable properties of chitosan have been reported to contribute to the delivery of nucleic acids to the lungs through the respiratory tract [105]. For example, Silva et al. showed that chitosan and siRNA powders prepared using carbon dioxide-assisted spraying with dry drying (SASD) technique effectively deposited in the mouse lungs after administration [168]. Okamoto et al. also used chitosan as a carrier to deliver pDNA as a dry powder. They found that chitosan as a dry powder resulted in higher expression of pDNA compared to intravenous or intratracheal infusion administration [169].
However, the strong electrostatic interactions between chitosan and nucleic acids can pose a challenge in achieving high transfection efficiency compared to other polymeric materials. To obtain higher nucleic acid delivery efficiency, chitosan-derived nanocarriers need to be developed to improve deacetylation degree, molecular weight, particle size, and N/P molar ratio [170, 171]. One possible solution to this problem is incorporating negatively charged compounds, such as γ-Polyglutamic acid (γ-PGA), into chitosan-nucleic acid complexes [172]. It was found that guanylated chitosan (GCS) promotes cellular uptake of siRNA and safely improves gene silencing efficiency. Further studies showed that the chemical coupling of salbutamol to GCS (SGCS) improved the targeting of siRNA nanoparticles to lung cells containing β2-adrenergic receptors [173]. Capel et al. modified chitosan with piperazine substitution. They showed that using modified chitosan as a carrier could mediate effective lung drug deposition after intratracheal administration and enhance siRNA-induced gene silencing in lung epithelial cells [174]. In a separate study, Jin et al. used imidazole ring-modified allantoic acid-modified chitosan as an aerosol delivery vehicle. They found that this modification resulted in higher gene transfection efficiency [175].
Klausner’s group used NOVAFECT chitosan (a currently commercially available chitosan designed by Arturrson [176]) for gene delivery studies. NOVAFECT chitosan-DNA nanoparticles injected into rat corneas showed specific expression of the luciferase gene in corneal fibroblasts and a 5.4-fold increase in expression compared to injection of polyethyleneimine-DNA nanoparticles [177]. Cheng and co-workers synthesized and applied galactosylated chitosan (GC) to encapsulate plasmids encoding macrophage colony-stimulating factor (GM-SCF) and interleukin (IL)-21. They found that following intravenous injection of GC/GM-CSF-IL-21 nanoparticles, GC/GM-CSF-IL-21 nanoparticles specifically accumulated in the liver and activated natural killer (NK) cells and cytolytic T-lymphocytes (CTLs) in tumor tissues of mice with liver metastasis model of colon cancer [178]. Similarly, Xiao et al. synthesized galactosylated chitosan-hydroxypropyltrimethylammonium (gal-HTCC) with galactosylated and quaternised chitosan. In vitro gene transfection results showed that gal-HTCC delivered pGL3 luciferase plasmid targeting to human hepatocellular carcinomas (HepG2) with remarkably higher transfection efficiencies (7–32-fold) compared with chitosan and gal-chitosan [179].
Poly-β-amino-ester (PBAE)
Due to the inherent cytotoxicity of PEI, there is a growing focus on developing polymers that retain the advantages of PEI but are easily degradable. One such cationic polymer is Poly-β-amino-ester (PBAE) (Fig. 4a), which has gained attention recently for its potential in the pulmonary delivery of nucleic acids. PBAE exhibits a highly adaptable chemical structure and is readily biodegradable. It can be synthesized by the Michael addition reaction [180]. The first attempt to use PBAE as a nucleic acid delivery vector was reported by Langer's team. They assembled pDNA with PBAE and found that the resulting complexes possessed a preferred nano-size and low cytotoxicity [181]. Later, Daniel G. Anderson and Robert Langer’s team explored the relationship between the capacity of pDNA-PBAE to overcome cellular barriers and the physicochemical properties of the materials [182]. They observed that the branched structure of PBAE was essential for enhancing the efficiency of nucleic acid delivery [180]. Concretely speaking, branched polymers demonstrated high transfection efficiency due to their three-dimensional (3D) spatial structure with multiple end groups [183].
The transfection efficiency of PBAE was also affected by the form of the end groups, such as end oligopeptides [184]. Anderson's group designed and synthesized a hyperbranched PBAE polymer, hDD90-118, that efficiently delivers nucleic acids to lungs by nebulization. Specifically, they delivered mRNA encoding luciferase using hDD90-118 and observed an even distribution of nanoparticles in all lung lobes. In the Ai14 reporter mouse model, they observed efficient transfection of lung epithelial cells [114] (Fig. 4b). Santangelo's group further investigated the use of hDD90-118 in nebulized delivery of nucleic acids to the lung. hDD90-118 was employed to deliver mRNA encoding virus-specific CRISPR-Cas13a protein or membrane-anchored neutralizing antibody [119, 124]. In one study, they used hDD90-118 to deliver mRNA encoding Cas13a to the respiratory tracts of mice and hamsters by nebulization, which resulted in efficient virus degradation and attenuation of respiratory infections [124]. In another experiment, the delivered cargo was exchanged for mRNA encoding a membrane-anchored neutralizing antibody. This led to the efficient expression of antibodies that alleviated the infection in the lungs of the experimental animals [119]. Afterward, they designed 166 new hyperbranched PBAE or Poly-β-amino-thio-ester (hPBATE) polymers using hDD90-118 as a precursor. Among these polymers, they found that at least five polymers, including P76, outperformed hDD90-118 in mediating lung mRNA expression. In therapeutic models of viral infections in hamsters, ferrets, cows, and non-human primates, P76 showed improved delivery efficiency in the pulmonary delivery of nucleic acids [123].
Generally, most nebulized or other inhalation methods target lung epithelial cells. Targeting pulmonary vascular endothelial cells also holds great potential in treating acute and chronic lung diseases, such as pulmonary hypertension and alveolar capillary dysplasia. In recent years, Anderson’s team has successfully achieved targeted delivery of mRNA to lung endothelial cells by combining PBAE with lipids in the form of nanoparticles (narrated in the corresponding section) [185, 186]. Modification of PBAE using lysine/histidine oligopeptides is also a promising approach. Dosta et al. found that high levels of gene silencing were observed in the pulmonary vascular endothelium after intravenous injection of PBAE-siRNA nanocomplexes, significantly reducing featureless delivery to organs such as the liver [187]. Kalinichenko’s group successfully achieved endothelial targeting after intravenous injection by unique structural design (altering the ratio of the two alkyl chains in the backbone and PEGylation and fluoride modification) of PBAE. They found that this modified PBAE could efficiently deliver pDNA to lung microcapillaries following intravenous administration [154] (Fig. 4c).
Kim et al. found that the structure of PBAE affects the biodistribution of nano complexes and gene transfection efficiency after intravenous injection. Also, they discovered by high-throughput barcode screening that PBAE NPs accumulated in the liver and spleen within 30 min of administration [188]. To target APCs in the spleen, Fornaguera et al. used oligopeptide-terminated modified PBAEs (OM-PBAEs) to deliver mRNA. They found that this newly designed material was able to accumulate efficiently in the spleen and was hardly affected by freeze-drying [189]. In a similar approach, Palmiero et al. utilized a strategy based on the synthesis of copolymers using polycaprolactone (PCL) and poly (beta-amino ester) (PBAE) through ring-opening and Michael addition polymerizations (PCL-based PBAE). They discovered that the selected ternary polymer exhibited significantly higher transfection efficiency compared to polyethyleneimine (PEI). The developed polymer primarily accumulated in the spleen with improved biocompatibility [190]. Zamboni et al. found that a primary formulation (Polymer 536, polymer to DNA weight ratio of 25) effectively transfected human Hepatocellular carcinoma (HCC) cell lines with a superior transfection efficiency over PEI and other commercially available transfection reagents (Lipofectamine™ 2000 and jetPRIME™). Notably, this biodegradable, end-modified PBAE gene delivery vector was non-cytotoxic [191]. Vaughan et al. administered PBAE nanoparticles via hepatic artery injection in a rat model of liver tumors. They found that arterial injection of PBAEs increased targeted transfection of HCC tumors compared to intravenous administration, making it a potential alternative method to Transcatheter Arterial Chemoembolization (TACE) [192]. Green's team used PBAE to deliver transgenes intracranially to enable specific gene expression in mouse gliomas. They found that the nanoparticles didn’t lose their potency after two years of freeze-drying and storage [193]. In another study, Mastorakos et al. showed that PEGylation PBAE nanoparticles delivering DNA rapidly penetrated healthy brain parenchyma and orthotopic brain tumor tissues. The nanoparticles significantly improved the survival in two aggressive orthotopic brain tumor models in rats [194].
Lipid-based delivery system
Cationic lipids
Cationic lipids have a permanent positive charge due to the covalent binding of a positively charged head group (such as a quaternary ammonium salt, an amine, a guanidine, or a heterocyclic compound) to a hydrophobic tail group through linkages chains [195, 196] (Fig. 5). In 1987, Felgner et al. synthesized cationic lipid 1,2-di-O-octadecenyl-3-trimethylpropylammonium (DOTMA) and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), which exhibited superior DNA loading capacity and efficient gene expression in vitro. It marks a pivotal moment for the application of cationic lipids in the field of nucleic acid delivery [197]. Since then, numerous research groups have embarked on employing cationic lipids as nucleic acid delivery vectors. It has been demonstrated that after intravenous injection, lipoplexes, positively charged complexes formed by cationic lipids and siRNA, can electrostatically interact with negatively charged erythrocytes to form agglomerates [198]. These agglomerates facilitate the adsorption of lipoplexes in highly dilated pulmonary capillaries, resulting in 60–70% drug accumulation in the lung within a few seconds after intravenous administration [199]. In a clinical trial, the successful treatment of patients with non-small cell lung cancer (NSCLC) was achieved through the delivery of pDNA encoding the tumor suppressor gene TUSC2/FUS1 using liposomes consisting of DOTAP and cholesterol [200]. In one study, positively charged ichthyoglobulin was used to form a complex with mRNA, and the resulting complex was further encapsulated with 1,2-dioleoyl-3-trimethylammonium propane (DOTAP). The prepared nanopcomplex induced robust cellular immune responses and delayed tumor growth [201]. High-density and low-molecular-weight PEGs have been applied to shield the surface charge of cationic lipids [202, 203]. The mucus barrier is the primary obstacle for nanoparticles delivering nucleic acids to the lung via inhalation. Taratula et al. achieved effective cell death induction and target gene silencing by co-delivering siRNA and chemotherapeutic drugs in the lung through nebulized inhalation using nanoparticles prepared with PEG-coated cationic lipid DOTAP. Also, with LHRH peptide modification of nanoparticles, they found that the drug was mainly enriched in lung tumors [204].
Cationic lipid-modified aminoglycoside (CLA) was explored to deliver mRNA to the liver. The results showed that a typical CLA named GT-EP10, delivering luciferase mRNA to mice at 0.05 mg/kg, achieved an average luminescence intensity of 107 in the liver [205]. Woitok et al. produced clinically functional LNP that contained the cationic amino-lipid KL52 using a T-junction. This LNP delivering c-Jun N-terminal kinase-2 (Jnk2) siRNA achieved efficient accumulation in the liver, and Jnk2 silencing ultimately reduced carcinogenesis in a model of advanced hepatocellular carcinoma [206]. Hattori et al. developed a novel method for siRNA transfer to the liver via intravenous injection of an anionic polymer and a cationic liposome/cholesterol-modified siRNA complex (cationic liposome). They found that siRNA accumulation shifted from the lungs to the liver when injected with poly L-glutamic acid (PGA) or chondroitin C (CS) sulfate [207]. Hsu and co-workers developed cationic nanoparticles, LNP-DP1, to deliver miR-122 for restoration of dysregulated gene expression in hepatocellular carcinoma (HCC) cells [208].
Afterward, novel cationic lipids with superior transfection efficiency were developed. Commercially available cationic lipids, such as Lipofectamine 2000, have been widely used for nucleic acid delivery owing to their superior encapsulation rate and transfection effect. For example, intratracheal injection of Lipofectamine 2000 delivering PAI-1 siRNA inhibited fibroblast proliferation and promoted apoptosis of fibroblasts in a rat model of bleomycin (BLM)-induced pulmonary fibrosis [209]. Johler and colleagues found that nebulized Lipofectamine 2000 nanoparticles encapsulating EGFP mRNA achieved a transfection rate of 38% in 16HBE cells, while polymer-based nanoparticles achieved a transfection rate of only 3% [210]. Another study found that intratracheal delivery of Rip2 siRNA using Lipofectamine 2000 suppressed indicators of cigarette smoke (CS)-induced inflammation and oxidative damage, as well as inhibiting the accumulation and transcriptional activation of nuclear p65 in lung tissues [211]. Mo et al. first reported that delivery of siRNA to Huh7.5 and H4IIE hepatocellular carcinoma cells using Lipofectamine 2000 increased autophagosome levels [212]. It should be noted that multivalent cationic lipids, such as Lipofectamine®, are more toxic than monovalent cationic lipids, such as DOTAP [213].
Another notable cationic lipid is GL67, considered the “gold standard” for respiratory non-viral gene delivery vectors. GL67 was first synthesized by LEE and co-workers as a cationic lipid with spermine as the head group, conjugated to cholesterol in a T-shape structure. GL67 efficiently delivered the plasmid encoding chloramphenicol acetyltransferase (CAT) to the lungs [214]. In another study, pDNA expressing the human cystic fibrosis transmembrane conductance regulator (CFTR) was delivered as an aerosol to sheep lungs using GL67A (a mixture of GL67/DOPE/DMPE-PEG5000) [215]. Recently, DACC formulation, composed of the b-L-arginyl-2,3-L-diaminopropionic acid N-palmitoyl-N-oleyl-amide trihydrochloride (AtuFECT01), cholesterol, mPEG2000-DSPE, facilitated efficient delivery of siRNA to lung tissues and reduced VE-calmodulin mRNA expression in the lungs by approximately 50% [216]. Moreover, gene expression can be silenced in pulmonary endothelial cells by a single-dose of DACC lipid complexes [217].
Ionizable lipids
Recently, ionizable lipids offer pH sensitivity to enhance nucleic acid delivery in vivo [218]. In physiological conditions with a pH of 7.4, ionizable lipids are electrically neutral and have reduced interactions with anionic cell membranes, rendering them biocompatible (Fig. 5). Moreover, ionizable lipids undergo ionization in endosomes where the pH is lower than that of the extracellular environment, resulting in a positive charge, which facilitates the escape of nanoparticles from the endosomes [219, 220]. In general, ionizable lipids are less toxic and more effective at endosomal escape than cationic lipids [221, 222]. The development of ionizable lipids began with the introduction of the first ionizable lipid, 1,2-dioleoyl-3-dimethylpropanaminium (DODAP), in which the head of a cationic lipid was replaced with an ionizable molecule [223]. Subsequently, Semple et al. formulated the first LNP consisting of DODAP, which achieved 70% encapsulation efficiency with oligonucleotides [224]. To further improve the encapsulation efficiency, the ionizable lipid 1,2-dilinoleyl-N, N-dimethyl-3-aminopropane (DLin-DMA) with a superior unsaturation degree was successfully synthesized [225]. Later, a more potent ionizable lipid 2,2-dilinoleyl-4-dimethylaminoethyl-1,3-dioxolane (DLin-KC2-DMA) was developed [226]. These previous studies have led to dilinoleylmethyl‐4‐dimethylaminobutyrate (DLin‐MC3‐DMA) with potent encapsulation efficiency and transfection capability. The head linker of DLin-MC3-DMA is an ester bond, making DLin-MC3-DMA biodegradable in vivo [227]. Onpattro®, a DLin-MC3-DMA-based nucleic acid drug, marks significant progress in the field of ionizable lipid molecules. Moreover, two mRNA vaccines (mRNA-1273 and BNT162b2) based on ionizable lipids received historic emergency use approvals, which may indicate a regulatory advantage for ionizable lipids [43, 228].
Drew Weissman’s group synthesized a lipid library of anisodamine ligands using a one-pot, two-step modular synthesis. The best-performing ionizable lipid, AA-T3A-C12, was able to silence heat shock protein 47 by ~ 65% and was twice as effective as the MC3 LNP in a preclinical model of liver fibrosis. AA-T3A-C12/siHSP47 LNP significantly reduced collagen deposition and alleviated liver fibrosis without significant toxicity [229]. Mounting evidence has demonstrated that LNP accumulates mainly in the liver after intravenous administration [230]. Therefore, there is a great challenge to design ionizable lipids for extrahepatic delivery. Kimura et al. first discovered that LNP prepared with DODAP and DOPE under a specific ratio could selectively deliver plasmid DNA to the spleen [231]. Recently, Zhang et al. designed a novel ionizable lipid for specific delivery of mRNA to the spleen. This lipid carried a positive charge under physiological conditions. It rapidly acquired a negative charge in the presence of esterases, thus allowing stabilization of mRNA encapsulation during storage and in vivo delivery while balancing effective mRNA release from the cytoplasm [232] (Fig. 6).
a The schematic of the lipid compounds (AMP-POC18) used in the article. b Transfection was assessed using AMB-POC18 LNPs encapsulating mRNA encoding luciferase and imaged using IVIS at different time points. c Detection of IFN-γ secretion using enzyme-linked immunospot analysis (ELISpot) after re-stimulation of splenocytes with SIINFEKL in vitro. d-g Antitumor effects of AMB-POC18-LNP loaded with mRNA encoding OVA as a therapeutic vaccine, evaluated by tumor volume, mouse survival, quantification of various types of immune cells, and tumor cell proliferation. h Lung metastases were recorded during the assessment of the therapeutic effect of vaccines on tumors by a laboratory model of tumor lung metastasis [232]. Copyright 2023, Wiley
To address biological barriers to nucleic acid delivery, Harashima and co-workers designed the first multifunctional envelope-type nano device (MEND) [233]. MEND is a nanoparticle that enables cell-specific targeting through surface modification by targeting ligands and incorporating pH-sensitive lipids. Pulmonary endothelial cell targeting is promising for treating various acute and chronic lung diseases [234]. Harashima’s group achieved targeted delivery to pulmonary endothelial cells by modifying a GALA peptide (developed initially as an endosomal destabilizer) on the surface of MEND [235, 236]. The same research group later designed a GALA-MEND incorporating a pH-sensitive lipid (YSK05) that efficiently delivered siRNA to pulmonary endothelial cells and inhibited lung cancer metastasis in mice. The addition of YSK05 improved the endosomal escape of MEND and enhanced the gene knock down effect in pulmonary endothelial cells [237]. Subsequently, they developed a dual-layer MEND delivery system with cell-penetrating peptide (R8 peptide). This system specifically delivered pDNA to macrophages and B cells in the spleen [238].
Xu and co-workers developed ionizable lipid 113-O12B targeting lymph nodes, which showed robust gene expression in lymph nodes. Targeted delivery of mRNA to lymph nodes increased CD8+ T-cell responses against a model antigen encoding ovalbumin (OVA) [239]. Most recently, the same group developed a unique LNP with a novel ionizable lipid containing an amide bond in the tail selectively delivered mRNA to the lungs. They synthesized a lipid library with amide bonds via a Michael addition reaction between the amine head and acrylamide tail and screened out the best lung-targeting lipid, 306-N16B. Interestingly, specific cell populations in the lungs can be targeted by simply switching the head structure of the LNP. In addition, through proteomics study, the researchers found that the abundant protein component in the 306-N16B corona was the fibrinogen beta chain. Then, they encapsulated mRNA encoding mouse tuberous sclerosis complex 2 (Tsc2) with 306-N16B. Following intravenous injection, the resulting nanocomplex restored the expression of the Tsc2 tumor suppressor for the treatment of lung lymphangioma (LAM) [109].
In a separate study, Daniel G. Anderson's team constructed OF-Deg-Lin lipids that induced 85% of protein expression in the spleen and effectively targeted B lymphocytes in vivo (~ 7%), showing the potential to modulate B cell function [240]. The same research team later synthesized 720 biodegradable lipids based on a three-component reaction system and screened out the top-performing ionizable lipid, RCB-4–8. In particular, the carbonate groups in RCB-4–8 rendered it more biodegradable than lipids with ester groups. They found that RCB-4–8 LNPs efficiently delivered mRNA to club cells and ciliated cells (the two significant subtypes of airway epithelial cells) via intratracheal delivery. Moreover, the pulmonary transfection efficiency of RCB-4–8 LNPs was superior over that of LNPs formulated with DLin-MC3-DMA, and the addition of the cationic lipid DOTAP further improved luciferase expression upon intratracheal administration. Also, they proposed a strategy for co-delivery of SpCas9 with adeno-associated virus (AAV) and RCB-4–8. Following intratracheal administration, immunostaining analysis of lung tissue sections showed activation of the tdTomato fluorescent reporter gene in 17.0 ± 5.0% of pulmonary cells [141].
Other lipids
In some studies, solid lipid nanoparticles (SLNs) were also employed for the pulmonary delivery of nucleic acids. Jacobson et al. demonstrated that siRNA-DOTAP complexes can be efficiently encapsulated within the neutral hydrophobic cores of SLNs using a hydrophobic ion-pair approach [241]. Recently, Wang and co-workers successfully delivered tumor necrosis factor-α (TNF-α) siRNA-SLNs by dry powder inhalation through a simulated mucus layer and achieved gene silencing in pulmonary macrophages and epithelial cells [242]. Most recently, Siegwart's group developed selective organ targeting (SORT) technology, which modulates the molar composition and internal charge of LNPs by adding new lipid molecules to conventional four-component LNPs. The addition of the SORT molecule to the original four-component LNP formulation allows for liver, lung, and spleen-specific targeting. They found that when negatively charged 1,2-dioleoyl-sn-glycero-3-phosphate (18PA) was added at 10–40%, SORT LNPs selectively accumulated and induced protein expression in the spleen, with no luciferase expression in other organs. Optimal lung delivery was achieved when DOTAP was added up to 50%, and complete liver targeting was reached by adding 20% DODAP. Based on SORT, ReCode has developed an inhalable mRNA vaccine (RCT1100) against Primary Ciliary Dyskinesia (PCD) that has entered Phase I clinical studies. The vaccine showed high DNAI1 protein expression, rapid LNP clearance in ciliated, rod, and basal cells, excellent tolerability, and the potential for repeated administration [147]. The same group later synthesized a novel LNP delivery system called iPLNPs, which consist of novel phospholipids (iPhos) with endosomal escape properties. They found that adding DDAB to the top-performing iPhos 9A1P9 effectively mediated enhanced mRNA delivery and CRISPR-Cas9 gene editing in the lungs [243].
Polymer–lipid hybrid delivery system
Polymer–lipid hybrid nanoparticles may improve the safety and long-lasting efficacy of single nanomaterial for nucleic acid delivery by taking advantage of the complementary properties of polymer and lipid nanoparticles [151, 154, 187, 188]. Thanki and colleagues developed a lipid-polymer hybrid nanoparticle by incorporating poly(DL-lactic-co-glycolic acid) (PLGA) with DOTAP for siRNA delivery. Compared to DOTAP alone, this hybrid nanoparticle effectively increased the siRNA payload and greatly optimized the gene-silencing effect [244, 245]. Meyer et al. designed self-assembled lipid/polymer hybrid (LPH) nanoparticles of PLGA with DOTAP or MC3. It was demonstrated that LPH nanoparticles accumulated predominantly in the liver post intravenous administration, whereas luciferase proteins were specifically expressed in the spleen and lungs [246]. Matsumoto et al. designed PEI lipopolyplexes with DOTMA and pDNA. The charge ratios of the complexes to pDNA were calculated from the molar values of the nitrogen of PEI and the nitrogen of DOTMA to pDNA phosphate. As the charge ratio was four, the lipopolyplexes selectively expressed the gene in the spleen [247].
Recently, Philip J. Santangelo's group developed a novel polymer–lipid hybrid nanoparticle named NLD1 using DOTAP in conjunction with 7C1 (a low molecular weight PEI) [248]. Nebulized NLD1 mRNA encoding broadly neutralizing antibodies targeting haemagglutinin substantially prevented lethal H1N1 influenza virus infection in mice, with higher delivery efficiency compared to MC3 and cKK-E12 [249]. Anderson's research team demonstrated that intravenous injection of PBAE delivering mRNA remarkably accumulated in the lungs of mice [186]. Moreover, they found that adding PEG-lipid to PBAE reduced the nanoparticle size and further increased its specificity towards the lungs [185]. The resulting nanoparticles efficiently delivered Cre mRNA to pulmonary endothelial cells and immune cells. Recently, the same group optimized the properties of PBAE by incorporating a third hydrophobic alkylamine monomer to form a terpolymer. This copolymer, D90-C12-103, achieved DNA transfection with 1–2 orders of magnitude higher efficacy than other transfection reagents such as C12-200 and PEI [250] (Table 2).
Outlook and conclusion
Initially, viral vectors were utilized for nucleic acid delivery. However, their potential immunogenicity and safety concerns have posed insurmountable challenges, leading to a gradual shift towards synthetic nanocarriers. Recently, polymers have been one of the pioneering nanomaterials employed for the delivery of nucleic acids. Although polymers possess the advantages of easy synthesis and variable chemical structures, they often face restrictions in terms of biodegradability [251, 252]. Undoubtedly, the most successful nucleic acid carriers in the current stage are LNPs. A growing body of studies has confirmed that LNPs have been the most advanced nucleic acid delivery systems to date. They have gained extensive usage, including two approved mRNA vaccines on the market against SARS-CoV-2. Nevertheless, conventional LNPs are difficult to specifically target extrahepatic organs [55]. So far, the focus has shifted to designing delivery systems that can effectively deliver therapeutic agents to targeted tissues and cells [253]. Researchers have found that the tissue selectivity of nanoparticles is closely related to factors such as the surface charge of lipid fractions. Although cationic lipid-based LNPs consisting of cations, such as DOTAP, have achieved pulmonary delivery of nucleic acids to a certain extent, there are still some potential safety hazards associated with the unavoidable cytotoxicity of positively charged substances in vivo [251]. The emergence of ionizable lipids, which carry a positive charge only in a specific physiological environment, avoids the toxicity of cationic lipids and brings superior transfection efficiency [38, 222, 254,255,256,257,258]. Currently, most lung-targeted nucleic acid products that have entered the clinical research stage have been developed based on ionizable lipids, including MRT5005® RCT1100®. Recent studies have explored new approaches, such as attaching peptides or antibodies on the surface of nanoparticles, to achieve active targeting. The researchers have investigated the properties of materials that influence delivery efficacy and intend to provide solutions to overcome the limitations of existing materials [259].
We must acknowledge that existing technologies still cannot solve all problems, partly due to the challenges in material synthesis and design, and partly due to the factors determining the targeted effects of materials not being fully elucidated [260]. The key to developing next-generation nucleic acid delivery vectors with optimal properties lies on overcoming complex physiological barriers. Some strategies have been applied to overcome physiological barriers, such as the blood–brain barrier (BBB) [261]. The characteristics of nanoparticles, such as particle size and surface charge, play vital roles in overcoming the physiological barriers. When developing innovative nanomaterials, it is crucial to comprehensively assess their characteristics, including cytotoxicity, nucleic acid loading capacity, endosomal escape efficiency, and storage stability. A thorough exploration of the relationship between material properties and tissue targeting ability should be conducted. Most recently, high-throughput screening methods, such as DNA barcoding technology, have been employed to explore the active targeting ability of nanomaterials [262, 263]. The past decade has witnessed the accelerating development of nanomaterials for nucleic acid delivery. We envision that with the rapid development of biomaterial science, nucleic acid drugs will play central roles in the prevention and treatment of various diseases in the near future.
Availability of data and materials
Not applicable.
References
Zhao YX, Shu R, Liu J. The development and improvement of ribonucleic acid therapy strategies. Mol Ther-Nucl Acids. 2021;26:997–1013. https://doi.org/10.1016/j.omtn.2021.09.002.
Curreri A, Sankholkar D, Mitragotri S, et al. RNA therapeutics in the clinic. Bioeng Transl Med. 2022;8(1):e10374. https://doi.org/10.1002/btm2.10374.
Dammes N, Peer D. Paving the Road for RNA Therapeutics. Trends Pharmacol Sci. 2020;41(10):755–75. https://doi.org/10.1016/j.tips.2020.08.004.
Sridharan K, Gogtay NJ. Therapeutic nucleic acids: current clinical status. Br J Clin Pharmacol. 2016;82(3):659–72. https://doi.org/10.1111/bcp.12987.
Sarli SL, Watts JK. Harnessing nucleic acid technologies for human health on earth and in space. Life Sci Space Res. 2022;35:113–26. https://doi.org/10.1016/j.lssr.2022.08.006.
Kulkarni JA, Witzigmann D, Thomson SB, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630–43. https://doi.org/10.1038/s41565-021-00898-0.
Wu L, Zhou W, Lin L, et al. Delivery of therapeutic oligonucleotides in nanoscale. Bioact Mater. 2021;7:292–323. https://doi.org/10.1016/j.bioactmat.2021.05.038.
Yamada Y. Nucleic acid drugs-current status, issues, and expectations for exosomes. Cancers. 2021;13(19):19. https://doi.org/10.3390/cancers13195002.
Chen JX, Zhu DD, Lian BP, et al. Cargo-selective and adaptive delivery of nucleic acid therapeutics by bola-amphiphilic dendrimers. Proc Natl Acad Sci U S A. 2023;120(21):11. https://doi.org/10.1073/pnas.2220787120.
Damase TR, Sukhovershin R, Boada C, et al. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol. 2021;9:24. https://doi.org/10.3389/fbioe.2021.628137.
Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–30. https://doi.org/10.1038/nrd892.
Wu SY, Lopez-Berestein G, Calin GA, et al. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6(240):7. https://doi.org/10.1126/scitranslmed.3008362.
Finan C, Gaulton A, Kruger FA, et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9(383):15. https://doi.org/10.1126/scitranslmed.aag1166.
Kumar SRP, Markusic DM, Biswas M, et al. Clinical development of gene therapy: results and lessons from recent successes. MolTher-Methods Clin Dev. 2016;3:11. https://doi.org/10.1038/mtm.2016.34.
Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics - developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80. https://doi.org/10.1038/nrd4278.
Yu AM, Tu MJ. Deliver the promise: RNAs as a new class of molecular entities for therapy and vaccination. Pharmacol Ther. 2022;230:19. https://doi.org/10.1016/j.pharmthera.2021.107967.
Kazemian P, Yu SY, Thomson SB, et al. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol Pharm. 2022;19(6):1669–86. https://doi.org/10.1021/acs.molpharmaceut.1c00916.
Li ZH, Zhao WC, Ma SX, et al. A chemical-enhanced system for CRISPR-Based nucleic acid detection. Biosens Bioelectron. 2021;192:10. https://doi.org/10.1016/j.bios.2021.113493.
Patel S, Athirasala A, Menezes PP, et al. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng Part A. 2019;25(1–2):91–112. https://doi.org/10.1089/ten.tea.2017.0444.
Li J, Zeng HM, Li LW, et al. Biomembrane-wrapped gene delivery nanoparticles for cancer therapy. Front Bioeng Biotechnol. 2023;11:17. https://doi.org/10.3389/fbioe.2023.1211753.
Wadhwa A, Aljabbari A, Lokras A, et al. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):27. https://doi.org/10.3390/pharmaceutics12020102.
Dogbey DM, Torres VES, Fajemisin E, et al. Technological advances in the use of viral and non-viral vectors for delivering genetic and non-genetic cargos for cancer therapy. Drug Deliv Transl Res. 2023;13(11):2719–38. https://doi.org/10.1007/s13346-023-01362-3.
Escors D, Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch Immunol Ther Exp. 2010;58(2):107–19. https://doi.org/10.1007/s00005-010-0063-4.
Frank SB, Schulz VV, Miranti CK. A streamlined method for the design and cloning of shRNAs into an optimized Dox-inducible lentiviral vector. BMC Biotechnol. 2017;17:10. https://doi.org/10.1186/s12896-017-0341-x.
Zhao ZM, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioeng Transl Med. 2022;7(1):20. https://doi.org/10.1002/btm2.10258.
Shirley JL, de Jong YP, Terhorst C, et al. Immune responses to viral gene therapy vectors. Mol Ther. 2020;28(3):709–22. https://doi.org/10.1016/j.ymthe.2020.01.001.
Chiu W, Lin TY, Chang YC, et al. An update on gene therapy for inherited retinal dystrophy: experience in leber congenital amaurosis clinical trials. Int J Mol Sci. 2021;22(9):24. https://doi.org/10.3390/ijms22094534.
Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6(1):601–21. https://doi.org/10.1146/annurev-virology-092818-015530.
Bucher K, Rodríguez-Bocanegra E, Dauletbekov D, et al. Immune responses to retinal gene therapy using adeno-associated viral vectors-Implications for treatment success and safety. Prog Retin Eye Res. 2021;83:25. https://doi.org/10.1016/j.preteyeres.2020.100915.
Ertl HCJ. Immunogenicity and toxicity of AAV gene therapy. Front Immunol. 2022;13:9. https://doi.org/10.3389/fimmu.2022.975803.
Gresele P, Momi S, Marcucci R, et al. Interactions of adenoviruses with platelets immune thrombotic thrombocytopenia syndrome. Haematologica. 2021;106(12):3034–45. https://doi.org/10.3324/haematol.2021.279289.
Schmidt-Wolf GD, Schmidt-Wolf IGH. Non-viral and hybrid vectors in human gene therapy: an update. Trends Mol Med. 2003;9(2):67–72. https://doi.org/10.1016/s1471-4914(03)00005-4.
Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–55. https://doi.org/10.1038/nrg3763.
Ferreras LAB, Chan SY, Reina SV, et al. Rapidly Transducing and spatially localized magnetofection using peptide-mediated non-viral gene delivery based on iron oxide nanoparticles. ACS Appl Nano Mater. 2021;4(1):167–81. https://doi.org/10.1021/acsanm.0c02465.
Zu H, Gao DC. Non-viral vectors in gene therapy: recent development, challenges, and prospects. Aaps J. 2021;23(4):12. https://doi.org/10.1208/s12248-021-00608-7.
Shukla A, Maiti P. Nanomedicine and versatile therapies for cancer treatment. MedComm. 2022;3(3):e163. https://doi.org/10.1002/mco2.163.
Paunovska K, Loughrey D, Dahlman JE. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23(5):265–80. https://doi.org/10.1038/s41576-021-00439-4.
Tenchov R, Bird R, Curtze AE, et al. Lipid nanoparticles-from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–7015. https://doi.org/10.1021/acsnano.1c04996.
Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379(1):11–21. https://doi.org/10.1056/NEJMoa1716153.
Zhang XP, Goel V, Attarwala H, et al. Patisiran pharmacokinetics, pharmacodynamics, and exposure-response analyses in the phase 3 APOLLO trial in patients with Hereditary Transthyretin-Mediated (hATTR) amyloidosis. J Clin Pharmacol. 2020;60(1):37–49. https://doi.org/10.1002/jcph.1480.
Adams D, Polydefkis M, González-Duarte A, et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021;20(1):49–59. https://doi.org/10.1016/s1474-4422(20)30368-9.
González-Duarte A, Berk JL, Quan DN, et al. Analysis of autonomic outcomes in APOLLO, a phase III trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. J Neurol. 2020;267(3):703–12. https://doi.org/10.1007/s00415-019-09602-8.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2022;35(6):947–51. https://doi.org/10.1177/08971900211009650.
Ilyichev AA, Orlova LA, Sharabrin SV, et al. mRNA technology as one of the promising platforms for the SARS-CoV-2 vaccine development. Vavilovskii Zhurnal Genet Sel. 2020;24(7):802–7. https://doi.org/10.18699/vj20.676.
Zhang CL, Maruggi G, Shan H, et al. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:13. https://doi.org/10.3389/fimmu.2019.00594.
Wang Y, Zhang Z, Luo J, et al. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20(1):33. https://doi.org/10.1186/s12943-021-01311-z.
Yue J, Liu Y, Zhao M, et al. The R&D landscape for infectious disease vaccines. Nat Rev Drug Discovery. 2023. https://doi.org/10.1038/d41573-023-00119-4.
Nabel GJ. The future of gene therapy workshop on human gene therapy - current opportunities and future trends. Berkeley: Springer-Verlag, Berlin; 2003.
Priyanka, Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis. 2023;52:3. https://doi.org/10.1016/j.tmaid.2023.102550.
Tian YY, Deng ZY, Yang PH. mRNA vaccines: A novel weapon to control infectious diseases. Front Microbiol. 2022;13:16. https://doi.org/10.3389/fmicb.2022.1008684.
Huang YK, Zhu XR, Guo X, et al. Advances in mRNA vaccines for viral diseases. J Med Virol. 2023;95(7):20. https://doi.org/10.1002/jmv.28924.
Gu YZ, Duan JY, Yang N, et al. mRNA vaccines in the prevention and treatment of diseases. MedComm. 2022;3(3):31. https://doi.org/10.1002/mco2.167.
Friedrich M, Aigner A. Therapeutic siRNA: state-of-the-art and future perspectives. BioDrugs. 2022;36(5):549–71. https://doi.org/10.1007/s40259-022-00549-3.
Holm A, Lovendorf MB, Kauppinen S. Development of siRNA therapeutics for the treatment of liver diseases. Methods Mol Biol. 2021;2282:57–75. https://doi.org/10.1007/978-1-0716-1298-9_5.
Bennett J. Overview of Retinal Gene Therapy: Current Status and Future Challenges. Cold Spring Harb Perspect Med. 2023;13(7):9. https://doi.org/10.1101/cshperspect.a041278.
Liu GW, Guzman EB, Menon N, et al. Lipid nanoparticles for nucleic acid delivery to endothelial cells. Pharm Res. 2023;40(1):3–25. https://doi.org/10.1007/s11095-023-03471-7.
Arevalo CP, Bolton MJ, Le Sage V, et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science. 2022;378(6622):899–904. https://doi.org/10.1126/science.abm0271.
Cacicedo ML, Weinl-Tenbruck C, Frank D, et al. Phenylalanine hydroxylase mRNA rescues the phenylketonuria phenotype in mice. Front Bioeng Biotechnol. 2022;10:14. https://doi.org/10.3389/fbioe.2022.993298.
Yamazaki K, Kubara K, Ishii S, et al. Lipid nanoparticle-targeted mRNA formulation as a treatment for ornithine-transcarbamylase deficiency model mice. Mol Ther-Nucl Acids. 2023;33:210–26. https://doi.org/10.1016/j.omtn.2023.06.023.
Balian A, Hernandez FJ. Nucleases as molecular targets for cancer diagnosis. Biomark Res. 2021;9(1):16. https://doi.org/10.1186/s40364-021-00342-4.
Xu L, Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci. 2011;100(1):38–52. https://doi.org/10.1002/jps.22243.
Mahmoudi M, Landry MP, Moore A, et al. The protein corona from nanomedicine to environmental science. Nat Rev Mater. 2023;8(7):422–38. https://doi.org/10.1038/s41578-023-00552-2.
Ritz S, Schöttler S, Kotman N, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromol. 2015;16(4):1311–21. https://doi.org/10.1021/acs.biomac.5b00108.
Rampado R, Crotti S, Caliceti P, et al. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “stealthy” nanomaterials. Front Bioeng Biotechnol. 2020;8:19. https://doi.org/10.3389/fbioe.2020.00166.
Lundqvist M, Augustsson C, Lilja M, et al. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS One. 2017;12(4):15. https://doi.org/10.1371/journal.pone.0175871.
Shi D, Beasock D, Fessler A, et al. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180: 114079. https://doi.org/10.1016/j.addr.2021.114079.
Evangelopoulos M, Parodi A, Martinez JO, et al. Cell source determines the immunological impact of biomimetic nanoparticles. Biomaterials. 2016;82:168–77. https://doi.org/10.1016/j.biomaterials.2015.11.054.
He XY, Wang J, Tang YQ, et al. Recent advances of emerging spleen-targeting nanovaccines for immunotherapy. Adv Healthc Mater. 2023:18. https://doi.org/10.1002/adhm.202300351.
Zhang MM, Gao S, Yang DJ, et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm Sin B. 2021;11(8):2265–85. https://doi.org/10.1016/j.apsb.2021.03.033.
Sharma RK, Calderon C, Vivas-Mejia PE. Targeting Non-coding RNA for glioblastoma therapy: the challenge of overcomes the blood-brain barrier. Front Med Technol. 2021;3:18. https://doi.org/10.3389/fmedt.2021.678593.
Aly AEE, Waszczak BL. Intranasal gene delivery for treating Parkinson’s disease: overcoming the blood-brain barrier. Expert Opin Drug Deliv. 2015;12(12):1923–41. https://doi.org/10.1517/17425247.2015.1069815.
Chan TG, Morse SV, Copping MJ, et al. Targeted delivery of DNA-Au nanoparticles across the blood-brain barrier using focused ultrasound. ChemMedChem. 2018;13(13):1311–4. https://doi.org/10.1002/cmdc.201800262.
Pangeni R, Meng T, Poudel S, et al. Airway mucus in pulmonary diseases: muco-adhesive and muco-penetrating particles to overcome the airway mucus barriers. Int J Pharm. 2023;634:17. https://doi.org/10.1016/j.ijpharm.2023.122661.
Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61(2):75–85. https://doi.org/10.1016/j.addr.2008.09.008.
Ernst M, John T, Guenther M, et al. A model for the transient subdiffusive behavior of particles in mucus. Biophys J. 2017;112(1):172–9. https://doi.org/10.1016/j.bpj.2016.11.900.
Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thoracic Society. 2018;15:S143–8. https://doi.org/10.1513/AnnalsATS.201802-128AW.
Friis KP, Gracin S, Oag S, et al. Spray dried lipid nanoparticle formulations enable intratracheal delivery of mRNA. J Control Release. 2023;363:389–401. https://doi.org/10.1016/j.jconrel.2023.09.031.
Bai X, Zhao GL, Chen QJ, et al. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci Adv. 2022;8(25):18. https://doi.org/10.1126/sciadv.abn7162.
d’Angelo I, Costabile G, Durantie E, et al. Hybrid lipid/polymer nanoparticles for pulmonary delivery of siRNA: development and fate upon in vitro deposition on the human epithelial airway barrier. J Aerosol Med Pulm Drug Deliv. 2018;31(3):170–81. https://doi.org/10.1089/jamp.2017.1364.
Kim N, Duncan GA, Hanes J, et al. Barriers to inhaled gene therapy of obstructive lung diseases: a review. J Control Release. 2016;240:465–88. https://doi.org/10.1016/j.jconrel.2016.05.031.
De Backer L, Cerrada A, Pérez-Gil J, et al. Bio-inspired materials in drug delivery: exploring the role of pulmonary surfactant in siRNA inhalation therapy. J Control Release. 2015;220:642–50. https://doi.org/10.1016/j.jconrel.2015.09.004.
Rudolph C, Lausier J, Naundorf S, et al. In vivo gene delivery to the lung using polyethylenimine and fractured poly amidoamine dendrimers. J Gene Med. 2000;2(4):269–78. https://doi.org/10.1002/1521-2254(200007/08)2:4%3c269::Aid-jgm112%3e3.0.Co;2-f.
Hu M, Li X, You Z, et al. Physiological barriers and strategies of lipid-based nanoparticles for nucleic acid drug delivery. Adv Mater (Deerfield Beach, Fla). 2023:e2303266. https://doi.org/10.1002/adma.202303266.
Torres-Vanegas JD, Cruz JC, Reyes LH. Delivery systems for nucleic acids and proteins: barriers, cell capture pathways and nanocarriers. Pharmaceutics. 2021;13(3):38. https://doi.org/10.3390/pharmaceutics13030428.
Kubota K, Onishi K, Sawaki K, et al. Effect of the nanoformulation of siRNA-lipid assemblies on their cellular uptake and immune stimulation. Int J Nanomed. 2017;12:5121–33. https://doi.org/10.2147/ijn.S136426.
Jarallah SJ, Aldossary AM, Tawfik EA, et al. GL67 lipid-based liposomal formulation for efficient siRNA delivery into human lung cancer cells. Saudi Pharm J. 2023;31(7):1139–48. https://doi.org/10.1016/j.jsps.2023.05.017.
Chu S, Tang C, Yin CH. Effects of mannose density on in vitro and in vivo cellular uptake and RNAi efficiency of polymeric nanoparticles. Biomaterials. 2015;52:229–39. https://doi.org/10.1016/j.biomaterials.2015.02.044.
Suberi A, Grun MK, Mao T, et al. Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination. Sci Transl Med. 2023;15(709):eabq0603. https://doi.org/10.1126/scitranslmed.abq0603.
Hatakeyama H, Akita H, Ito E, et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials. 2011;32(18):4306–16. https://doi.org/10.1016/j.biomaterials.2011.02.045.
Zeng J, Shirihai OS, Grinstaff MW. Modulating lysosomal pH: a molecular and nanoscale materials design perspective. J Life Sci (Westlake Village, Calif). 2020;2(4):25–37. https://doi.org/10.36069/jols/20201204.
Hu YW, Carraro-Lacroix LR, Wang A, et al. Lysosomal pH plays a key role in regulation of mTOR activity in osteoclasts. J Cell Biochem. 2016;117(2):413–25. https://doi.org/10.1002/jcb.25287.
Rayamajhi S, Marchitto J, Nguyen TDT, et al. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloid Surf B-Biointerfaces. 2020;188:9. https://doi.org/10.1016/j.colsurfb.2020.110804.
Hoekstra D, Rejman J, Wasungu L, et al. Gene delivery by cationic lipids: in and out of an endosome. Biochem Soc Trans. 2007;35:68–71. https://doi.org/10.1042/bst0350068.
Spencer DS, Shodeinde AB, Beckman DW, et al. Biodegradable cationic nanogels with tunable size, swelling and pKa for drug delivery. Int J Pharm. 2020;588:8. https://doi.org/10.1016/j.ijpharm.2020.119691.
Gyanani V, Goswami R. Key design features of lipid nanoparticles and electrostatic charge-based lipid nanoparticle targeting. Pharmaceutics. 2023;15(4):33. https://doi.org/10.3390/pharmaceutics15041184.
Zuhorn IS, Bakowsky U, Polushkin E, et al. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005;11(5):801–10. https://doi.org/10.1016/j.ymthe.2004.12.018.
Somiya M, Liu Q, Kuroda SI. Current Progress of Virus-mimicking Nanocarriers for Drug Delivery. Nanotheranostics. 2017;1(4):415–29. https://doi.org/10.7150/ntno.21723.
Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjugate Chem. 2008;19(4):920–7. https://doi.org/10.1021/bc700448h.
Alipour M, Hosseinkhani S, Sheikhnejad R, et al. Nano-biomimetic carriers are implicated in mechanistic evaluation of intracellular gene delivery. Sci Rep. 2017;7:13. https://doi.org/10.1038/srep41507.
Ols S, Yang L, Thompson EA, et al. Route of vaccine administration alters antigen trafficking but not innate or adaptive immunity. Cell Rep. 2020;30(12):3964-71.e7. https://doi.org/10.1016/j.celrep.2020.02.111.
Hansen S, Lehr CM. Nanoparticles for transcutaneous vaccination. Microb Biotechnol. 2012;5(2):156–67. https://doi.org/10.1111/j.1751-7915.2011.00284.x.
Xue L, Yan YF, Kos P, et al. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv Transl Res. 2021;11(1):255–60. https://doi.org/10.1007/s13346-020-00790-9.
Merkel OM, Beyerle A, Librizzi D, et al. Nonviral siRNA delivery to the lung: investigation of PEG-PEI polyplexes and their in vivo performance. Mol Pharm. 2009;6(4):1246–60. https://doi.org/10.1021/mp900107v.
Howard KA, Rahbek UL, Liu XD, et al. RNA interference in vitro and in vivo using a chitosan/siRNA nanoparticle system. Mol Ther. 2006;14(4):476–84. https://doi.org/10.1016/j.ymthe.2006.04.010.
Sharma K, Somavarapu S, Colombani A, et al. Nebulised siRNA encapsulated crosslinked chitosan nanoparticles for pulmonary delivery. Int J Pharm. 2013;455(1–2):241–7. https://doi.org/10.1016/j.ijpharm.2013.07.024.
Saunders NRM, Paolini MS, Fenton OS, et al. A Nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020;20(6):4264–9. https://doi.org/10.1021/acs.nanolett.0c00752.
Lee HY, Mohammed KA, Peruvemba S, et al. Targeted lung cancer therapy using ephrinA1-loaded albumin microspheres. J Pharm Pharmacol. 2011;63(11):1401–10. https://doi.org/10.1111/j.2042-7158.2011.01306.x.
Qiu M, Tang Y, Chen JJ, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119(8):10. https://doi.org/10.1073/pnas.2116271119.
Germershaus O, Nultsch K. Localized, non-viral delivery of nucleic acids: opportunities, challenges and current strategies. Asian J Pharm Sci. 2015;10(3):159–75. https://doi.org/10.1016/j.ajps.2014.10.001.
Deng ZC, Kalin GT, Shi DL, et al. Nanoparticle delivery systems with cell-specific targeting for pulmonary diseases. Am J Respir Cell Mol Biol. 2021;64(3):292–307. https://doi.org/10.1165/rcmb.2020-0306TR.
Zoulikha M, Xiao QQ, Boafo GF, et al. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12(2):600–20. https://doi.org/10.1016/j.apsb.2021.08.009.
Kubczak M, Michlewska S, Bryszewska M, et al. Nanoparticles for local delivery of siRNA in lung therapy. Adv Drug Deliv Rev. 2021;179:19. https://doi.org/10.1016/j.addr.2021.114038.
Patel AK, Kaczmarek JC, Bose S, et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv Mater. 2019;31(8):7. https://doi.org/10.1002/adma.201805116.
Chow MYT, Qiu YS, Lam JKW. Inhaled RNA therapy: from promise to reality. Trends Pharmacol Sci. 2020;41(10):715–29. https://doi.org/10.1016/j.tips.2020.08.002.
Del Sorbo L, Costamagna A, Muraca G, et al. Intratracheal administration of small interfering RNA targeting fas reduces lung ischemia-reperfusion injury. Crit Care Med. 2016;44(8):E604–13. https://doi.org/10.1097/ccm.0000000000001601.
Lei H, Alu A, Yang J, et al. Intranasal administration of a recombinant RBD vaccine induces long-term immunity against Omicron-included SARS-CoV-2 variants. Signal Transduct Target Ther. 2022;7(1):159. https://doi.org/10.1038/s41392-022-01002-1.
Tiwari PM, Vanover D, Lindsay KE, et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat Commun. 2018;9:15. https://doi.org/10.1038/s41467-018-06508-3.
Vanover D, Zurla C, Peck HE, et al. Nebulized mRNA-encoded antibodies protect hamsters from SARS-CoV-2 infection. Adv Sci. 2022;9(34):13. https://doi.org/10.1002/advs.202202771.
Laube BL. Aerosolized medications for gene and peptide therapy. Respir Care. 2015;60(6):806–21. https://doi.org/10.4187/respcare.03554.
Farkas A, Tomisa G, Szénasi G, et al. The effect of lung emptying before the inhalation of aerosol drugs on drug deposition in the respiratory system. Int J Pharm X. 2023;6:7. https://doi.org/10.1016/j.ijpx.2023.100192.
Smith J, Tiner R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;378(9795):982–1082. https://doi.org/10.1016/s0140-6736(11)61445-1.
Rotolo L, Vanover D, Bruno NC, et al. Species-agnostic polymeric formulations for inhalable messenger RNA delivery to the lung. Nat Mater. 2023;22(3):369–79. https://doi.org/10.1038/s41563-022-01404-0.
Blanchard EL, Vanover D, Bawage SS, et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat Biotechnol. 2021;39(6):717–26. https://doi.org/10.1038/s41587-021-00822-w.
Gottlieb J, Zamora MR, Hodges T, et al. ALN-RSV01 for prevention of bronchiolitis obliterans syndrome after respiratory syncytial virus infection in lung transplant recipients. J Heart Lung Transplant. 2016;35(2):213–21. https://doi.org/10.1016/j.healun.2015.08.012.
Miao H, Huang K, Li YW, et al. Optimization of formulation and atomization of lipid nanoparticles for the inhalation of mRNA. Int J Pharm. 2023;640:11. https://doi.org/10.1016/j.ijpharm.2023.123050.
Kularatne RN, Crist RM, Stern ST. The future of tissue-targeted lipid nanoparticle-mediated nucleic acid delivery. Pharmaceuticals. 2022;15(7):17. https://doi.org/10.3390/ph15070897.
Witzigmann D, Uhl P, Sieber S, et al. Optimization-by-design of hepatotropic lipid nanoparticles targeting the sodium-taurocholate cotransporting polypeptide. Elife. 2019;8:e42276. https://doi.org/10.7554/eLife.42276.
Debacker AJ, Voutila J, Catley M, et al. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol Ther. 2020;28(8):1759–71. https://doi.org/10.1016/j.ymthe.2020.06.015.
Ding C, Li YY, Guo FF, et al. A cell-type-resolved liver psroteome. Mol Cell Proteomics. 2016;15(10):3190–202. https://doi.org/10.1074/mcp.M116.060145.
Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25(2):195–206. https://doi.org/10.1016/j.bpg.2011.02.005.
Freitas-Lopes MA, Mafra K, David BA, et al. Differential location and distribution of hepatic immune cells. Cells. 2017;6(4):22. https://doi.org/10.3390/cells6040048.
van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10(9):664-U24. https://doi.org/10.1038/nri2832.
Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4(33):eaau6085. https://doi.org/10.1126/sciimmunol.aau6085.
Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806–18. https://doi.org/10.1016/j.immuni.2013.10.010.
Santacroce L, Charitos IA, Ballini A, et al. The human respiratory system and its microbiome at a glimpse. Biology-Basel. 2020;9(10):16. https://doi.org/10.3390/biology9100318.
Manisalidis I, Stavropoulou E, Stavropoulos A, et al. Environmental and health impacts of air pollution: a review. Front Public Health. 2020;8:13. https://doi.org/10.3389/fpubh.2020.00014.
Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. https://doi.org/10.1016/s0140-6736(22)00478-0.
Hossain MM, Nesa F, Das J, et al. Global burden of mental health problems among children and adolescents during COVID-19 pandemic: an umbrella review. Psychiatry Res. 2022;317:16. https://doi.org/10.1016/j.psychres.2022.114814.
Yang J, Gong YH, Zhang CE, et al. Co-existence and co-infection of influenza A viruses and coronaviruses: public health challenges. Innovation-Amsterdam. 2022;3(5):10. https://doi.org/10.1016/j.xinn.2022.100306.
Li BW, Manan RS, Liang SQ, et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat Biotechnol. 2023:12. https://doi.org/10.1038/s41587-023-01679-x.
Zarogouldis P, Karamanos NK, Porpodis K, et al. Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int J Mol Sci. 2012;13(9):10828–62. https://doi.org/10.3390/ijms130910828.
Gavitt TD, Hartmann AK, Sawant SS, et al. A GATA3 targeting nucleic acid nanocapsule for in vivo gene regulation in asthma. ACS Nano. 2021;15(7):11192–201. https://doi.org/10.1021/acsnano.0c07781.
McCarthy SD, Rohde CB, Angel M, et al. Aerosolized pulmonary delivery of mRNA constructs attenuates severity of escherichia coli pneumonia in the rat. Nucl Acid Ther. 2023;33(2):148–58. https://doi.org/10.1089/nat.2022.0049.
Ahn I, Kang CS, Han J. Where should siRNAs go: applicable organs for siRNA drugs. Exp Mol Med. 2023;55(7):1283–92. https://doi.org/10.1038/s12276-023-00998-y.
Kauffman KJ, Oberli MA, Dorkin JR, et al. Rapid, single-cell analysis and discovery of vectored mRNA transfection in vivo with a loxP-flanked tdTomato reporter mouse. Mol Ther-Nucl Acids. 2018;10:55–63. https://doi.org/10.1016/j.omtn.2017.11.005.
Cheng Q, Wei T, Farbiak L, et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313–20. https://doi.org/10.1038/s41565-020-0669-6.
Leong EWX, Ge RW. Lipid nanoparticles as delivery vehicles for inhaled therapeutics. Biomedicines. 2022;10(9):25. https://doi.org/10.3390/biomedicines10092179.
Jere D, Jiang HL, Arote R, et al. Degradable polyethylenimines as DNA and small interfering RNA carriers. Expert Opin Drug Deliv. 2009;6(8):827–34. https://doi.org/10.1517/17425240903029183.
Mann JFS, McKay PF, Arokiasamy S, et al. Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. J Control Release. 2013;170(3):452–9. https://doi.org/10.1016/j.jconrel.2013.06.004.
Günther M, Lipka J, Malek A, et al. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011;77(3):438–49. https://doi.org/10.1016/j.ejpb.2010.11.007.
Piotrowski-Daspit AS, Kauffman AC, Bracaglia LG, et al. Polymeric vehicles for nucleic acid delivery. Adv Drug Deliv Rev. 2020;156:119–32. https://doi.org/10.1016/j.addr.2020.06.014.
Lv H, Zhu Q, Liu KW, et al. Coupling of a bifunctional peptide R13 to OTMCS-PEI copolymer as a gene vector increases transfection efficiency and tumor targeting. Int J Nanomed. 2014;9:1311–22. https://doi.org/10.2147/ijn.S59726.
Deng ZC, Gao W, Kohram F, et al. Fluorinated amphiphilic Poly(β-Amino ester) nanoparticle for highly efficient and specific delivery of nucleic acids to the Lung capillary endothelium. Bioact Mater. 2024;31:1–17. https://doi.org/10.1016/j.bioactmat.2023.07.022.
Mao SR, Neu M, Germershaus O, et al. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjugate Chem. 2006;17(5):1209–18. https://doi.org/10.1021/bc060129j.
Sufianov A, Beilerli A, Kudriashov V, et al. Advances in transdermal siRNAs delivery: a review of current research progress. Non-Coding RNA Res. 2023;8(3):392–400. https://doi.org/10.1016/j.ncrna.2023.05.008.
Beyerle A, Braun A, Merkel O, et al. Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J Control Release. 2011;151(1):51–6. https://doi.org/10.1016/j.jconrel.2010.12.017.
Lipka J, Semmler-Behnke M, Wenk A, et al. Biokinetic datasets of PEI F25-LMW complexed and non-complexed 32P-siRNA within different lung compartments. Data Brief. 2016;7:1175–8. https://doi.org/10.1016/j.dib.2016.03.092.
Lipka J, Semmler-Behnke M, Wenk A, et al. Biokinetic studies of non-complexed siRNA versus nano-sized PEI F25-LMW/siRNA polyplexes following intratracheal instillation into mice. Int J Pharm. 2016;500(1–2):227–35. https://doi.org/10.1016/j.ijpharm.2016.01.038.
de Oliveira FA, Albuquerque LJC, Nascimento-Sales M, et al. Balancing gene transfection and cytotoxicity of nucleic acid carriers with focus on ocular and hepatic disorders: evaluation of hydrophobic and hydrophilic polyethyleneimine derivatives. J Mat Chem B. 2023;11(20):4556–71. https://doi.org/10.1039/d3tb00477e.
Kurosaki T, Nakasone C, Kodama Y, et al. Splenic gene delivery system using self-assembling nano-complex with phosphatidylserine analog. Biol Pharm Bull. 2015;38(1):23–9. https://doi.org/10.1248/bpb.b14-00478.
Yang L, Wang T, Zhang D, et al. Black phosphorus nanosheets assist nanoerythrosomes for efficient mRNA vaccine delivery and immune activation. Adv Healthc Mater. 2023;12(26): e2300935. https://doi.org/10.1002/adhm.202300935.
Aranaz I, Alcántara AR, Civera MC, et al. Chitosan: an overview of its properties and applications. Polymers. 2021;13(19):27. https://doi.org/10.3390/polym13193256.
Jiménez-Gómez CP, Cecilia JA. Chitosan: a natural biopolymer with a wide and varied range of applications. Molecules. 2020;25(17):43. https://doi.org/10.3390/molecules25173981.
Naveed M, Phil L, Sohail M, et al. Chitosan oligosaccharide (COS): an overview. Int J Biol Macromol. 2019;129:827–43. https://doi.org/10.1016/j.ijbiomac.2019.01.192.
Dmour I, Islam N. Recent advances on chitosan as an adjuvant for vaccine delivery. Int J Biol Macromol. 2022;200:498–519. https://doi.org/10.1016/j.ijbiomac.2021.12.129.
Cao Y, Tan YF, Wong YS, et al. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17(6):21. https://doi.org/10.3390/md17060381.
Silva AS, Shopsowitz KE, Correa S, et al. Rational design of multistage drug delivery vehicles for pulmonary RNA interference therapy. Int J Pharm. 2020;591:15. https://doi.org/10.1016/j.ijpharm.2020.119989.
Okamoto H, Shiraki K, Yasuda R, et al. Chitosan-interferon-β gene complex powder for inhalation treatment of lung metastasis in mice. J Control Release. 2011;150(2):187–95. https://doi.org/10.1016/j.jconrel.2010.12.006.
Cao Y, Tan YF, Wong YS, et al. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17(6):381. https://doi.org/10.3390/md17060381.
Rudzinski WE, Aminabhavi TM. Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm. 2010;399(1–2):1–11. https://doi.org/10.1016/j.ijpharm.2010.08.022.
Liao ZX, Ho YC, Chen HL, et al. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/siRNA complexes via the inclusion of a negatively charged poly(γ-glutamic acid). Biomaterials. 2010;31(33):8780–8. https://doi.org/10.1016/j.biomaterials.2010.07.086.
Luo YF, Zhai XY, Ma CN, et al. An inhalable β2-adrenoceptor ligand-directed guanidinylated chitosan carrier for targeted delivery of siRNA to lung. J Control Release. 2012;162(1):28–36. https://doi.org/10.1016/j.jconrel.2012.06.005.
Capel V, Vllasaliu D, Watts P, et al. Water-soluble substituted chitosan derivatives as technology platform for inhalation delivery of siRNA. Drug Deliv. 2018;25(1):644–53. https://doi.org/10.1080/10717544.2018.1440668.
Jin H, Kim TH, Hwang SK, et al. Aerosol delivery of urocanic acid-modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K-ras null mice. Mol Cancer Ther. 2006;5(4):1041–9. https://doi.org/10.1158/1535-7163.Mct-05-0433.
Köping-Höggård M, Vårum KM, Issa M, et al. Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers. Gene Ther. 2004;11(19):1441–52. https://doi.org/10.1038/sj.gt.3302312.
Klausner EA, Zhang Z, Chapman RL, et al. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials. 2010;31(7):1814–20. https://doi.org/10.1016/j.biomaterials.2009.10.031.
Cheng M, Li Q, Wan T, et al. Synthesis and efficient hepatocyte targeting of galactosylated chitosan as a gene carrier in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2011;99(1):70–80. https://doi.org/10.1002/jbm.b.31873.
Xiao B, Wang X, Qiu Z, et al. A dual-functionally modified chitosan derivative for efficient liver-targeted gene delivery. J Biomed Mater Res A. 2013;101(7):1888–97. https://doi.org/10.1002/jbm.a.34493.
Nezhad MS. Poly (beta-amino ester) as an in vivo nanocarrier for therapeutic nucleic acids. Biotechnol Bioeng. 2023;120(1):95–113. https://doi.org/10.1002/bit.28269.
Lynn DM, Langer R. Degradable poly(β-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–8. https://doi.org/10.1021/ja0015388.
Akinc A, Lynn DM, Anderson DG, et al. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003;125(18):5316–23. https://doi.org/10.1021/ja034429c.
Cordeiro RA, Serra A, Coelho JFJ, et al. Poly(β-amino ester)-based gene delivery systems: from discovery to therapeutic applications. J Control Release. 2019;310:155–87. https://doi.org/10.1016/j.jconrel.2019.08.024.
Riera R, Tauler J, Feiner-Gracia N, et al. Complex pBAE nanoparticle cell trafficking: tracking both position and composition using super resolution microscopy. ChemMedChem. 2022;17(13):8. https://doi.org/10.1002/cmdc.202100633.
Kaczmarek JC, Kauffman KJ, Fenton OS, et al. Optimization of a degradable polymer-lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 2018;18(10):6449–54. https://doi.org/10.1021/acs.nanolett.8b02917.
Kaczmarek JC, Patel AK, Kauffman KJ, et al. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew Chem-Int Edit. 2016;55(44):13808–12. https://doi.org/10.1002/anie.201608450.
Dosta P, Demos C, Ramos V, et al. Delivery of siRNA to endothelial cells in vivo using lysine/histidine oligopeptide-modified Poly(β-amino ester) nanoparticles. Cardiovasc Eng Technol. 2021;12(1):114–25. https://doi.org/10.1007/s13239-021-00518-x.
Kim J, Vaughan HJ, Zamboni CG, et al. High-throughput evaluation of polymeric nanoparticles for tissue-targeted gene expression using barcoded plasmid DNA. J Control Release. 2021;337:105–16. https://doi.org/10.1016/j.jconrel.2021.05.047.
Fornaguera C, Guerra-Rebollo M, Ángel Lázaro M, et al. mRNA delivery system for targeting antigen-presenting cells in vivo. Adv Healthc Mater. 2018;7(17): e1800335. https://doi.org/10.1002/adhm.201800335.
Capasso Palmiero U, Kaczmarek JC, Fenton OS, et al. Poly(β-amino ester)-co-poly(caprolactone) Terpolymers as Nonviral Vectors for mRNA Delivery In Vitro and In Vivo. Adv Healthc Mater. 2018;7(14): e1800249. https://doi.org/10.1002/adhm.201800249.
Zamboni CG, Kozielski KL, Vaughan HJ, et al. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. J Control Release. 2017;263:18–28. https://doi.org/10.1016/j.jconrel.2017.03.384.
Vaughan HJ, Zamboni CG, Luly KM, et al. Non-viral gene delivery to hepatocellular carcinoma via intra-arterial injection. Int J Nanomedicine. 2023;18:2525–37. https://doi.org/10.2147/ijn.S390384.
Guerrero-Cázares H, Tzeng SY, Young NP, et al. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014;8(5):5141–53. https://doi.org/10.1021/nn501197v.
Mastorakos P, Zhang C, Song E, et al. Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors. J Control Release. 2017;262:37–46. https://doi.org/10.1016/j.jconrel.2017.07.009.
Ghosh YK, Visweswariah SS, Bhattacharya S. Nature of linkage between the cationic headgroup and cholesteryl skeleton controls gene transfection efficiency. FEBS Lett. 2000;473(3):341–4. https://doi.org/10.1016/s0014-5793(00)01558-1.
Zhang Y, Sun C, Wang C, et al. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121(20):12181–277. https://doi.org/10.1021/acs.chemrev.1c00244.
Felgner PL, Gadek TR, Holm M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84(21):7413–7. https://doi.org/10.1073/pnas.84.21.7413.
Eliyahu H, Servel N, Domb AJ, et al. Lipoplex-induced hemagglutination: potential involvement in intravenous gene delivery. Gene Ther. 2002;9(13):850–8. https://doi.org/10.1038/sj.gt.3301705.
Simberg D, Weisman S, Talmon Y, et al. The role of organ vascularization and lipoplex-serum initial contact in intravenous murine lipofection. J Biol Chem. 2003;278(41):39858–65. https://doi.org/10.1074/jbc.M302232200.
Lu C, Stewart DJ, Lee JJ, et al. Phase I clinical trial of systemically administered TUSC2 (FUS1)-nanoparticles mediating functional gene transfer in humans. PLoS One. 2012;7(4):9. https://doi.org/10.1371/journal.pone.0034833.
Mai YP, Guo JS, Zhao Y, et al. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354:9. https://doi.org/10.1016/j.cellimm.2020.104143.
Xu QG, Ensign LM, Boylan NJ, et al. Impact of surface Polyethylene Glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution n vivo. ACS Nano. 2015;9(9):9217–27. https://doi.org/10.1021/acsnano.5b03876.
Lai SK, O’Hanlon DE, Harrold S, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A. 2007;104(5):1482–7. https://doi.org/10.1073/pnas.0608611104.
Taratula O, Kuzmov A, Shah M, et al. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–57. https://doi.org/10.1016/j.jconrel.2013.04.018.
Yu X, Liu S, Cheng Q, et al. Lipid-Modified Aminoglycosides for mRNA Delivery to the Liver. Adv Healthc Mater. 2020;9(7): e1901487. https://doi.org/10.1002/adhm.201901487.
Woitok MM, Zoubek ME, Doleschel D, et al. Lipid-encapsulated siRNA for hepatocyte-directed treatment of advanced liver disease. Cell Death Dis. 2020;11(5):343. https://doi.org/10.1038/s41419-020-2571-4.
Hattori Y, Arai S, Okamoto R, et al. Sequential intravenous injection of anionic polymer and cationic lipoplex of siRNA could effectively deliver siRNA to the liver. Int J Pharm. 2014;476(1–2):289–98. https://doi.org/10.1016/j.ijpharm.2014.09.059.
Hsu SH, Yu B, Wang X, et al. Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine. 2013;9(8):1169–80. https://doi.org/10.1016/j.nano.2013.05.007.
Zhang YP, Li WB, Wang WL, et al. siRNA against plasminogen activator inhibitor-1 ameliorates bleomycin-induced lung fibrosis in rats. Acta Pharmacol Sin. 2012;33(7):897–908. https://doi.org/10.1038/aps.2012.39.
Johler SM, Rejman J, Guan S, et al. Nebulisation of IVT mRNA complexes for intrapulmonary administration. PLoS One. 2015;10(9):11. https://doi.org/10.1371/journal.pone.0137504.
Dong JR, Liao WP, Tan LH, et al. Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury. Pharmacol Res. 2019;139:560–8. https://doi.org/10.1016/j.phrs.2018.10.016.
Mo RH, Zaro JL, Ou JHJ, et al. Effects of lipofectamine 2000/sirna complexes on autophagy in hepatoma cells. Mol Biotechnol. 2012;51(1):1–8. https://doi.org/10.1007/s12033-011-9422-6.
Dokka S, Toledo D, Shi XG, et al. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17(5):521–5. https://doi.org/10.1023/a:1007504613351.
Lee ER, Marshall J, Siegel CS, et al. Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung. Hum Gene Ther. 1996;7(14):1701–17. https://doi.org/10.1089/hum.1996.7.14-1701.
McLachlan G, Davidson H, Holder E, et al. Pre-clinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther. 2011;18(10):996–1005. https://doi.org/10.1038/gt.2011.55.
Gutbier B, Kube SM, Reppe K, et al. RNAi-mediated suppression of constitutive pulmonary gene expression by small interfering RNA in mice. Pulm Pharmacol Ther. 2010;23(4):334–44. https://doi.org/10.1016/j.pupt.2010.03.007.
Fehring V, Schaeper U, Ahrens K, et al. Delivery of therapeutic siRNA to the lung endothelium via novel lipoplex formulation DACC. Mol Ther. 2014;22(4):811–20. https://doi.org/10.1038/mt.2013.291.
Carrasco MJ, Alishetty S, Alameh MG, et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun Biol. 2021;4(1):15. https://doi.org/10.1038/s42003-021-02441-2.
Cardarelli F, Pozzi D, Bifone A, et al. Cholesterol-dependent macropinocytosis and endosomal escape control the transfection efficiency of lipoplexes in CHO living cells. Mol Pharm. 2012;9(2):334–40. https://doi.org/10.1021/mp200374e.
Kowalski PS, Rudra A, Miao L, et al. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. 2019;27(4):710–28. https://doi.org/10.1016/j.ymthe.2019.02.012.
Sato Y. Development of lipid nanoparticles for the delivery of macromolecules based on the molecular design of pH-sensitive cationic lipids. Chem Pharm Bull. 2021;69(12):1141–59.
Yan Y, Liu XY, Lu A, et al. Non-viral vectors for RNA delivery. J Control Release. 2022;342:241–79. https://doi.org/10.1016/j.jconrel.2022.01.008.
Bailey AL, Cullis PR. Modulation of membrane-fusion by asymmetric transbilayer distributions of amino liPIDS. Biochemistry. 1994;33(42):12573–80. https://doi.org/10.1021/bi00208a007.
Semple SC, Klimuk SK, Harasym TO, et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim Biophys Acta-Biomembr. 2001;1510(1–2):152–66. https://doi.org/10.1016/s0005-2736(00)00343-6.
Heyes J, Palmer L, Bremner K, et al. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107(2):276–87. https://doi.org/10.1016/j.jconrel.2005.06.014.
Semple SC, Akinc A, Chen JX, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28(2):172-U18. https://doi.org/10.1038/nbt.1602.
Jayaraman M, Ansell SM, Mui BL, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem-Int Edit. 2012;51(34):8529–33. https://doi.org/10.1002/anie.201203263.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389.
Han XX, Gong NQ, Xue LL, et al. Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat Commun. 2023;14(1):12. https://doi.org/10.1038/s41467-022-35637-z.
Akinc A, Maier MA, Manoharan M, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14(12):1084–7. https://doi.org/10.1038/s41565-019-0591-y.
Kimura S, Khalil IA, Elewa YHA, et al. Novel lipid combination for delivery of plasmid DNA to immune cells in the spleen. J Control Release. 2021;330:753–64. https://doi.org/10.1016/j.jconrel.2021.01.005.
Zhang R, Shao S, Piao Y, et al. Esterase-labile quaternium lipidoid enabling improved mRNA-LNP stability and spleen-selective mRNA transfection. Adv Mater. 2023;35:e2303614. https://doi.org/10.1002/adma.202303614.
Kogure K, Akita H, Yamada Y, et al. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv Drug Deliv Rev. 2008;60(4–5):559–71. https://doi.org/10.1016/j.addr.2007.10.007.
Huertas A, Guignabert C, Barberà JA, et al. Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases. Eur Resp J. 2018;51(4):13. https://doi.org/10.1183/13993003.00745-2017.
Li WJ, Nicol F, Szoka FC. GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–85. https://doi.org/10.1016/j.addr.2003.10.041.
Kusumoto K, Akita H, Ishitsuka T, et al. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic siRNA delivery to the pulmonary endothelium. ACS Nano. 2013;7(9):7534–41. https://doi.org/10.1021/nn401317t.
Abd Elwakil MM, Khalil IA, Elewa YHA, et al. Lung-endothelium-targeted nanoparticles based on a pH-sensitive lipid and the GALA peptide enable robust gene silencing and the regression of metastatic lung cancer. Adv Funct Mater. 2019;29(18):13. https://doi.org/10.1002/adfm.201807677.
Kimura S, Khalil IA, Elewa YHA, et al. Spleen selective enhancement of transfection activities of plasmid DNA driven by octaarginine and an ionizable lipid and its implications for cancer immunization. J Control Release. 2019;313:70–9. https://doi.org/10.1016/j.jconrel.2019.09.009.
Chen JJ, Ye ZF, Huang CF, et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci U S A. 2022;119(34):10. https://doi.org/10.1073/pnas.2207841119.
Fenton OS, Kauffman KJ, Kaczmarek JC, et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv Mater. 2017;29(33):7. https://doi.org/10.1002/adma.201606944.
Jacobson GB, Gonzalez-Gonzalez E, Spitler R, et al. Biodegradable nanoparticles with sustained release of functional siRNA in skin. J Pharm Sci. 2010;99(10):4261–6. https://doi.org/10.1002/jps.22147.
Wang JL, Hanafy MS, Xu HY, et al. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int J Pharm. 2021;596:9. https://doi.org/10.1016/j.ijpharm.2021.120215.
Liu S, Cheng Q, Wei T, et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20(5):701–10. https://doi.org/10.1038/s41563-020-00886-0.
Thanki K, Zeng XH, Justesen S, et al. Engineering of small interfering RNA-loaded lipidoid-poly(DL-lactic-co-glycolic acid) hybrid nanoparticles for highly efficient and safe gene silencing: A quality by design-based approach. Eur J Pharm Biopharm. 2017;120:22–33. https://doi.org/10.1016/j.ejpb.2017.07.014.
Jose C, Amra K, Bhavsar C, et al. Polymeric lipid hybrid nanoparticles: properties and therapeutic applications. Crit Rev Ther Drug Carr Syst. 2018;35(6):555–88. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2018024751.
Meyer RA, Hussmann GP, Peterson NC, et al. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int J Pharm. 2022;611: 121314. https://doi.org/10.1016/j.ijpharm.2021.121314.
Matsumoto M, Kishikawa R, Kurosaki T, et al. Hybrid vector including polyethylenimine and cationic lipid, DOTMA, for gene delivery. Int J Pharm. 2008;363(1–2):58–65. https://doi.org/10.1016/j.ijpharm.2008.07.010.
Dahlman JE, Barnes C, Khan OF, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–55. https://doi.org/10.1038/nnano.2014.84.
Lokugamage MP, Vanover D, Beyersdorf J, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5(9):1059–68. https://doi.org/10.1038/s41551-021-00786-x.
Kaczmarek JC, Patel AK, Rhym LH, et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275:120966. https://doi.org/10.1016/j.biomaterials.2021.120966.
Lv HT, Zhang SB, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–9. https://doi.org/10.1016/j.jconrel.2006.04.014.
Kharwade R, Badole P, Mahajan N, et al. Toxicity and surface modification of dendrimers: a critical review. Curr Drug Deliv. 2022;19(4):451–65. https://doi.org/10.2174/1567201818666211021160441.
Chan WCW. Principles of nanoparticle delivery to solid tumors. BME Front. 2023;4:16.
Chen HL, Ren X, Xu S, et al. Optimization of lipid nanoformulations for effective mRNA delivery. Int J Nanomed. 2022;17:2893–905. https://doi.org/10.2147/ijn.S363990.
Hirai Y, Saeki R, Song FR, et al. Charge-reversible lipid derivative: a novel type of pH-responsive lipid for nanoparticle-mediated siRNA delivery. Int J Pharm. 2020;585:10. https://doi.org/10.1016/j.ijpharm.2020.119479.
Liu LX, Zhou YM, Xu AQ, et al. Non-Viral Nucleic Acid Delivery System for RNA Therapeutics. Adv Therap. 2023;6(8):23. https://doi.org/10.1002/adtp.202300005.
Zhang YQ, Guo RR, Chen YH, et al. Ionizable drug delivery systems for efficient and selective gene therapy. Military Med Res. 2023;10(1):29. https://doi.org/10.1186/s40779-023-00445-z.
Sun D, Lu ZR. Structure and function of cationic and ionizable lipids for nucleic acid delivery. Pharm Res. 2023;40(1):27–46. https://doi.org/10.1007/s11095-022-03460-2.
Zhang Z, Liu C, Li C, et al. Shape effects of cylindrical versus spherical unimolecular polymer nanomaterials on in vitro and in vivo behaviors. Research (Wash D C). 2019;2019:2391486. https://doi.org/10.34133/2019/2391486.
Lu Y, Zeng T, Zhang H, et al. Nano-immunotherapy for lung cancer. Nano TransMed. 2023;2(1):e9130018. https://doi.org/10.26599/NTM.2023.9130018.
Lee JH, Chapman DV, Saltzman WM. Nanoparticle Targeting with Antibodies in the Central Nervous System. BME Front. 2023;4:0012. https://doi.org/10.34133/bmef.0012
Guimaraes PPG, Zhang R, Spektor R, et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J Control Release. 2019;316:404–17. https://doi.org/10.1016/j.jconrel.2019.10.028.
Hatit MZC, Lokugamage MP, Dobrowolski CN, et al. Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat Nanotechnol. 2022;17(3):310–8. https://doi.org/10.1038/s41565-021-01030-y.
Acknowledgements
Some of the figures were created with Biorender.com
Funding
This study was supported by the National Natural Science Foundation of China (NSFC 82202311).
Author information
Authors and Affiliations
Contributions
Ling Li conceived the project. Yuyang Qin, Liyuan Ou and Ling Li prepared the manuscript. Yuyang Qin and Liyuan Ou created the diagrams. Lili Zha and Yue Zeng revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
This manuscript has not been published elsewhere before. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qin, Y., Ou, L., Zha, L. et al. Delivery of nucleic acids using nanomaterials. Mol Biomed 4, 48 (2023). https://doi.org/10.1186/s43556-023-00160-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43556-023-00160-0