Abstract
Background
The purpose of this study was to evaluate the hemogram parameters, namely NLR and PLR, at the end of the first year of antifibrotic treatment in IPF patients and evaluate the association of change in NLR and PLR levels and mortality in this study population. Patients diagnosed with IPF and started on antifibrotic therapy between 2016 and 2022 were included. Study design was retrospective cohort study. Baseline NLR and PLR values were obtained at the time of diagnosis before any treatment was started. Follow-up levels of NLR and PLR were obtained at the completion of the first year of antifibrotic treatment.
Results
A total of 125 patients were included in the study. Mean age was 67.9 ± 7.27 years. It was found that increment in NLR at first year was significant in non-survived group (p = 0.01). When patients were separated into four groups according to their survival status and antifibrotic medication, significant NLR and PLR elevations were only present in non-survived pirfenidone group (p = 0.02 and p = 0.01).
Conclusions
Elevated levels of NLR at the first year of antifibrotic treatment may be a sign of worse prognosis in IPF patients, especially in patients treated with pirfenidone.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive disease of lungs typically occurring in smoking males over the age of 50. Despite the use of two antifibrotic medications, nintedanib and pirfenidone, disease has significant impacts on quality of life and may progress causing respiratory failure with requirement of supplemental oxygen therapy and eventually necessitate lung transplantation [1]. Patients should be considered for early referral for lung transplantation because of the progressive nature of the disease. Therefore, it is essential to discriminate patients at risk of disease progression.
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are simple and accessible parameters of inflammation and oxidative stress, and in numerous studies, their high levels were correlated with worse prognosis of disease, like rheumatoid arthritis, chronic obstructive pulmonary disease (COPD), cancers, and IPF [2,3,4,5,6]. These parameters are usually evaluated by their levels at the time of the diagnosis. However, it is not clear whether repeated measures of these parameters are correlated with treatment response, disease severity, or mortality.
The purpose of this study was to evaluate the hemogram parameters, namely NLR and PLR, at the end of the first year of antifibrotic treatment in IPF patients and evaluate the association of change in NLR and PLR levels and mortality in this study population. Secondarily, it was aimed to investigate the change of these biomarkers according to the stage of disease.
Methods
This study was conducted in a tertiary level reference hospital, and local ethical committee approval was obtained (local ethical committee approval date and number: 01/06/2022 — (2022) 8–41). Informed consent of the patients was waived due to retrospective design of the study. Patients diagnosed with IPF and started on antifibrotic therapy between 2016 and 2022 were included in the study. IPF diagnosis was obtained according to the 2018 ATS/ERS diagnostic criteria and 2011 ATS/ERS diagnostic criteria for the patients diagnosed before 2018, either by lung biopsy or combined evaluation of clinical and high-resolution computed tomography characteristics [7, 8]. All patients were over the age of 18. Study design was retrospective cohort study. Patients with inadequate data, with diagnosis of other fibrotic diseases of lungs (like chronic hypersensitivity pneumonitis or connective tissue disease), and patients lost on follow-up were excluded. Patients who discontinued antifibrotic therapy before 1 year of treatment were also excluded.
Demographic, clinical, laboratory, and radiological characteristics were obtained from the hospital records. Age, sex, comorbidities, smoking status, and body mass index (BMI) data were collected. Survival status of the patients was obtained. Any treatment change or side effects during study period were noted. All patients had basal spirometry for measurement of forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC), with diffusion capacity of lungs for carbon monoxide (DLCO) and 6-min walk test (6MWT) for estimating functional capacity. Spirometry was performed according to the ATS/ERS statement guidelines. Diffusion capacity of the lungs was measured by single breath carbon monoxide uptake method. GAP (gender-age-physiology) index for individual patient was calculated according to the method suggested by Ley et al. [9].
Baseline NLR and PLR values were obtained at the time of diagnosis before any treatment was started. Follow-up levels of NLR and PLR were obtained at the completion of the first year of antifibrotic treatment. Antifibrotic choice, as either nintedanib or pirfenidone, was decided by the attending physician and multidisciplinary council. Mortality data was obtained at the follow-up period from the date of diagnosis to the start of the study.
Statistical analysis
SPSS (Statistical Package for Social Sciences) version 22 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Normal distribution of the variables was evaluated by Kolmogorov-Smirnov test and Shapiro-Wilk test. According to the distribution of data, values were given as mean and standard deviation or median and interquartile range. Comparison of the independent variables was achieved by independent samples t-test or Mann-Whitney U-test. Evaluation of repeated measures of NLR and PLR was accomplished by Wilcoxon signed-rank test. For multivariate analysis, patients were categorized according to the presence of NLR and PLR increments in their first-year evaluation. Backwards Wald method was used for multivariate analysis. p < 0.05 was taken as the level of significance, and type-1 error coefficient was determined as alpha 0.05.
Results
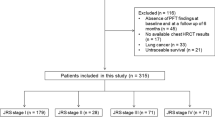
One-hundred thirty-seven IPF patients were screened for the study. A total of 125 patients with the diagnosis of idiopathic pulmonary fibrosis (IPF) were included in the study. Flow chart of the study population may be seen in Fig. 1. Mean age of the study population was 67.9 ± 7.27 years, with 80% males (n = 100). Diagnosis of IPF was achieved by histopathological confirmation either with lung cryobiopsy or video-associated lung surgical biopsy in 24% of the patients (n = 30). In the remaining 95 patients, diagnosis was obtained by clinical and tomographic features. Most of the patients had definite usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) scans (n = 92, 73.6%). Nineteen patients (15.2%) had concomitant emphysema. GAP stage of the study population was stage I in 40% (n = 50), stage 2 in 48% (n = 60), and stage 3 in 15 patients (12%).
All patients were given antifibrotic treatment, 57 patients (45.6%) were given nintedanib, while remaining 68 patients (54.4%) were on pirfenidone. In 10 patients (8%), progressive disease was evident. In 16 patients, treatment change was made either due to unresponsiveness or side effects, in 37 patients either dose was reduced, or treatment was stopped due to side effects.
Demographic, clinical, and laboratory characteristics of the study population are demonstrated in Table 1.
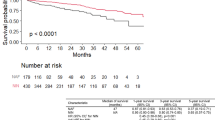
There were 72 deaths during the study period. Mean follow-up time for the whole population was 37.8 ± 19.02 months. Mean survival time was 28.0 ± 15.47 months. Percentage of males were similar in survived and non-survived groups (p = 1.00). Although the presence of at least one comorbidity was similar across groups (p = 0.21), all patients with lung cancer were in non-survived group (0 vs 6 (%8.3), p = 0.04). Clubbing of digits was significantly seen more in non-survived group, but difference was nonsignificant (p = 0.26). Body mass index of the groups was similar (p = 0.33); however, basal FVC and diffusion capacity of lungs were significantly lower in non-survived group (respectively p = 0.02 and p < 0.001). Among laboratory parameters, C-reactive protein (CRP) was significantly higher in non-survived group (p = 0.004).
NLR and PLR levels at the time of diagnosis and at the first year of the antifibrotic therapy were similar between survived and non-survived groups (for basal NLR and PLR, respectively, p = 0.70 and 0.87; for NLR and PLR at first year, respectively, p = 0.13 and p = 0.16).
However, for the total study population, change in NLR at first year was significantly higher from the basal NLR level (p = 0.03). Basal PLR levels and 1st year PLR levels were similar (p = 0.30). When survived and non-survived population were separately evaluated, it was found that increment in NLR at first year was significant in non-survived group (p = 0.01), whereas NLR levels were similar in survived patients (p = 0.82). The same situation was evident for PLR levels, but PLR increments at first year were at the edge of significance for non-survived population (p = 0.07). Changes of NLR and PLR levels at first year of antifibrotic therapy in survived and non-survived populations are presented in Table 2.
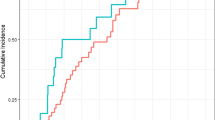
There was not any statistically significant difference of repeated measures of NLR and PLR in all GAP stages (for NLR, p = 0.41 in GAP stage I, p = 0.12 in GAP stage II, p = 0.6 in GAP stage III; for PLR, p = 0.63 in GAP stage I, p = 0.33 in GAP stage II, p = 0.18 in GAP stage III). However, NLR levels at diagnosis, NLR levels at first year, and PLR levels at first year were significantly elevated in GAP stage III patients (respectively, median 3.55 (interquartile range (IQR): 3.38) vs. median 2.3 (IQR 1.22), p = 0.02; median 5.44 (IQR: 4,73) vs. median 2.44 (IQR: 1.4) p = 0.01; and median 184.5 (IQR: 185.8) vs. median 124.8 (IQR: 58.9), p = 0.05).
Patients were evaluated according to the antifibrotic medication used, and in patients treated with pirfenidone, elevations in NLR and PLR levels at first year were significant, whereas it was nonsignificant in nintedanib group (in pirfenidone group, p = 0.004 for NLR and p = 0.007 for PLR; in nintedanib group, p = 0.97 for NLR and p = 0.16 for PLR). Data are presented in Table 3.
When patients were separated into four groups according to their survival status and antifibrotic medication, it was seen that significant NLR and PLR elevations were only present in non-survived pirfenidone group (respectively, p = 0.02 and p = 0.01). NLR and PLR change in these four groups is seen in Table 4.
Regarding the whole study population, multivariate analysis of possible predictive factors of mortality was carried out considering BMI, GAP index, the presence of comorbidities, smoking status, and the presence of NLR and PLR increments. GAP index was the only predictive factor remained in the equation of multivariate analysis (HR: 3.11, 95% CI: 1.44–6.71, p = 0.004).
Discussion
In this study, our results show a marked increase in NLR and PLR levels in non-survived patient population at the first year of antifibrotic treatment. This increment is prominent and significant in NLR levels and was not detected in survived group. Although NLR and PLR levels at first year were similar between survived and non-survived population, change of these levels according to pretreatment levels may give information about prognosis of IPF. This increment is mostly prominent in non-survived pirfenidone-treated subgroup.
NLR and PLR are simple, easily obtained biomarkers of oxidative stress and inflammation, first analyzed in critically ill patients with sepsis and systemic infection [2]. Beside acute illnesses and cancers, prognostic role of these simple markers was also evaluated in chronic conditions, like chronic obstructive pulmonary disease, chronic ischemic heart disease, and IPF [10,11,12]. However, change of these parameters during follow-up of IPF patients was investigated in a few studies.
Chen et al. found a significant association between baseline NLR levels and IPF mortality; higher NLR levels on admission were correlated with shorter survival time [13]. Also, NLR and PLR levels were negatively correlated with PaO2/FiO2 levels. In non-survived subgroup of their study population, they compared NLR, PLR, and MHR levels before death and in their last admission and found that NLR levels were significantly elevated compared with the baseline levels. However, this subgroup of patients had acute exacerbation of IPF causing inhospital mortality, and elevated NLR levels were associated with acute exacerbation-related deaths. In our patient population, eight patients had at least one acute exacerbation history, all of which were in non-survived group. However, NLR and PLR levels at first year were obtained during routine follow-up visit of patients.
In the post hoc analysis of double-blind trials of pirfenidone, Nathan et al. found that greatest change in NLR levels at 12 months, rather that PLR levels, was associated with worse outcome parameters, including mortality [14]. In contrast to our study, they found this association to be more prominent in placebo group, and the authors concluded that these biomarker changes may be less suitable for IPF patients given antifibrotic therapy. Our study population does not include treatment naive patients, and our results show more significant elevations of these markers in pirfenidone-treated subgroup. Studies shown immune modulatory effects of pirfenidone, while anti-inflammatory effects of nintedanib are less prominent [15,16,17,18]. The difference between pirfenidone and nintedanib groups may be related to these immunomodulatory effects of pirfenidone.
In the study of Zinellu et al., NLR and PLR levels, together with other blood indexes, were found to be elevated in IPF patients, and NLR levels were also correlated with FVC and DLCOlevels [19]. We also found that NLR levels at first year and at diagnosis and PLR levels at first year were significantly higher in GAP stage III patients as expected. However, we failed to show any significant difference between NLR and PLR levels at first year and at diagnosis according to disease stage.
One of the remarkable results of our study was that all patients with IPF and lung cancer were in the non-survived group. Previous studies have reported worse prognosis in IPF patients with lung cancer [20, 21]. In the study of Mohamed S. et al., lung cancer was one of the most significant predictors of survival in IPF patients with a hazard ratio of 5.43 [21]. Our results are in accordance with these findings.
There are some limitations of our study. Data are retrospectively collected, and study population is from a single center and limited for obtaining robust evidence. We were not able to perform multivariate analysis in subgroup of patients, due to limited number of patients.However, we found our results important as it represents data of IPF patients in antifibrotic era.
Conclusions
Beside from baseline levels, elevated levels of NLR at the first year of antifibrotic treatment may be a sign of worse prognosis in IPF patients, especially in patients treated with pirfenidone. Future studies are needed to elucidate the prognostic role changes of these simple parameters, NLR and PLR, and their association with treatment response or future acute exacerbation risk.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mei Q, Liu Z, Zuo H, Yang Z, Qu J (2021) Idiopathic pulmonary fibrosis: an update on pathogenesis. Front Pharmacol 12:797292
Zahorec R (2001) Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102(1):5–14
Erre GL, Paliogiannis P, Castagna F, Mangoni AA, Carru C, Passiu G, Zinellu A (2019) Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest 49(1):e13037
Ruta VM, Man AM, Alexescu TG, Motoc NS, Tarmure S, Ungur RA, Todea DA, Coste SC, Valean D, Pop MC (2020) Neutrophil-To-Lymphocyte Ratio and Systemic Immune-Inflammation Index-Biomarkers in Interstitial Lung Disease. Medicina (Kaunas) 56(8):381
Zinellu A, Zinellu E, Mangoni AA, Pau MC, Carru C, Pirina P, Fois AG (2022) Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev 31(166):220095
Portale G, Bartolotta P, Azzolina D, Gregori D, Fiscon V (2023) Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: systematic review and meta-analysis. Langenbecks Arch Surg 408(1):85
Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, Flaherty KR, Wells A, Martinez FJ, Azuma A, Bice TJ, Bouros D, Brown KK, Collard HR, Duggal A, Galvin L, Inoue Y, Jenkins RG, Johkoh T, Kazerooni EA, Kitaichi M, Knight SL, Mansour G, Nicholson AG, Pipavath SNJ, Buendía-Roldán I, Selman M, Travis WD, Walsh S, Wilson KC (2018) Diagnosis of idiopathic pulmonary fibrosis An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198(5):e44–e68
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ (2011) ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183(6):788–824
Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, King TE Jr, Collard HR (2012) A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 156(10):684–691
El-Gazzar AG, Kamel MH, Elbahnasy OKM, El-Naggar ME (2020) Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med 14(1):111–116
Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G, Zong G (2021) Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis 32(8):715–720
Mikolasch TA, George PM, Sahota J, Nancarrow T, Barratt SL, Woodhead FA, Kouranos V, Cope VSA, Creamer AW, Fidan S, Ganeshan B, Hoy L, Mackintosh JA, Shortman R, Duckworth A, Fallon J, Garthwaite H, Heightman M, Adamali HI, Lines S, Win T, Wollerton R, Renzoni EA, Steward M, Wells AU, Gibbons M, Groves AM, Gooptu B, Scotton CJ, Porter JC (2023) Multi-center evaluation of baseline neutrophil-to-lymphocyte (NLR) ratio as an independent predictor of mortality and clinical risk stratifier in idiopathic pulmonary fibrosis. EClinicalMedicine 55:101758
Chen Y, Cai J, Zhang M, Yan X (2022) Prognostic role of NLR, PLR and MHR in patients with idiopathic pulmonary fibrosis. Front Immunol 13:882217
Nathan SD, Mehta J, Stauffer J, Morgenthien E, Yang M, Limb SL, Bhorade S (2021) Changes in Neutrophil-Lymphocyte or Platelet-Lymphocyte Ratios and Their Associations with Clinical Outcomes in Idiopathic Pulmonary Fibrosis. J Clin Med 10(7):1427
Ozbilgin K, Üner MA, Ozkut M, Hasdemir PS (2017) The effects of pirfenidone on T helper cells in prevention of intraperitoneal adhesions. Kaohsiung J Med Sci 33(6):271–276
Visner GA, Liu F, Bizargity P, Liu H, Liu K, Yang J, Wang L, Hancock WW (2009) Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation 88(3):330–338
Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B (2014) Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 349(2):209–220
Iyer SN, Hyde DM, Giri SN (2000) Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation 24(5):477–491
Zinellu A, Paliogiannis P, Sotgiu E, Mellino S, Mangoni AA, Zinellu E, Negri S, Collu C, Pintus G, Serra A, Pistuddi AM, Carru C, Pirina P, Fois AG (2020) Blood cell count derived inflammation indexes in patients with idiopathic pulmonary fibrosis. Lung 198(5):821–827
Whittaker Brown SA, Padilla M, Mhango G, Taioli E, Powell C, Wisnivesky J (2019) Outcomes of older patients with pulmonary fibrosis and non-small cell lung cancer. Ann Am Thorac Soc 16(8):1034–1040
Mohamed S, Bayoumi H, El-Aziz NA, Mousa E, Gamal Y (2018) Prevalence, risk factors, and impact of lung cancer on outcomes of idiopathic pulmonary fibrosis: a study from the Middle East. Multidiscip Respir Med 3(13):37
Acknowledgements
Not applicable.
Funding
The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Author information
Authors and Affiliations
Contributions
Author OO and author GP have given substantial contributions to the conception or the design of the manuscript and author FDU, author DSU, author SE, and author TS to acquisition, analysis, and interpretation of the data. All authors have participated in drafting the manuscript, and author GP revised it critically. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in a tertiary level reference hospital, and local ethical committee approval was obtained (local ethical committee approval date and number: 01/06/2022 — (2022) 8–41). Informed consent of the patients was waived due to retrospective design of the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Özdemir, Ö., Polat, G., Üçsular, F.D. et al. Elevations in NLR at the first year of pirfenidone treatment may be associated with worse prognosis in patients with idiopathic pulmonary fibrosis. Egypt J Bronchol 18, 28 (2024). https://doi.org/10.1186/s43168-024-00280-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-024-00280-3