Abstract
Background
The wingless signaling pathway of bone development is inhibited by sclerostin, which may contribute to the etiology of ankylosing spondylitis.
Aim
The study aimed to evaluate serum sclerostin levels in ankylosing spondylitis patients and investigate how it correlated with radiographic damage using the Spondylo-arthritis Research Consortium of Canada index (SPARCC), disease activity, and functional impairment.
Results
This cross-sectional case–control study revealed a significantly lower mean serum sclerostin (11.28 ng/ml) in AS patients compared with controls (101.25 ng/ml). Serum sclerostin levels showed a significant negative correlation with each of Bath Ankylosing Spondylitis Metrology Index (BASMI) (p = 0.043), sacroiliac joints SPARCC, spine SPARCC, and overall SPARCC scores (p = 0.012, p = 0.036, and p = 0.007). The detection of AS, serum sclerostin levels ≤ 20 ng/ml showed 100% sensitivity and specificity.
Conclusion
Serum sclerostin had good discriminating power between ankylosing spondylitis cases and healthy control individuals and was correlated with subclinical activity status on magnetic resonance imaging.
Similar content being viewed by others
Background
Axial and peripheral entheseal inflammation associated with new bone formation are the hallmarks of ankylosing spondylitis (AS) [1]. The growth of syndesmophytes results in spinal fusion and functional disability [2]. It has recently been studied how the wingless signaling pathway (Wnt/-catenin pathway) and its inhibitors contribute to the pathophysiology of AS. The canonical Wnt pathway's initiation, results in the transcription of genes essential for osteoblast growth and the creation of new bone. Therefore, the decreased Wnt inhibitors or their impaired function might be involved in AS pathogenesis. Sclerostin is a secreted glycoprotein expressed by the SOST gene “the gene that provides instructions for making the protein sclerostin” [3]. Osteocytes and some chondrocytes predominantly generate it. Since it inhibits the Wnt signaling pathway, sclerostin has been shown to have anti-anabolic effects on the growth of new bone [4, 5].
The study aimed to assess the serum sclerostin level in patients with AS and look for any relationships with disease activity, radiographic damage, and functional impairment.
Patients and methods
This cross-sectional case–control study included twenty AS male patients aged 25 to 45 who met the Assessment of SpondyloArthritis International Society (ASAS) criteria for axial spondyloarthritis [6]. Twenty age-matched males served as controls. All participants provided written informed consent. The ethics committee approved the study.
Every participant underwent the following:
-
1)
Full medical history includes disease duration, degree of back pain using VAS, morning stiffness duration, and extraarticular manifestations (e.g., uveitis, and psoriasis).
-
2)
Clinical examination with special concern about skin, nails, hair, peripheral, and axial joint examination. BASMI was measured for spinal mobility [7].
-
3)
Evaluation of AS disease activity by Bath AS disease activity (BASDAI) [8] and Ankylosing Spondylitis Disease Activity Score (ASDAS) [9], functional status by Bath ankylosing spondylitis functional index (BASFI) [10].
-
4)
Comprehensive blood counts (CBC), erythrocyte sedimentation rates (ESR), C-reactive protein (CRP), and HLA-B27 were all measured in a lab setting.
-
5)
A modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) was used to perform and evaluate radiographs of the spine and pelvis [11]. The involvement of the sacroiliac joints was assessed using the New York criteria [12].
-
6)
Both sacroiliac joints (SIJs) underwent magnetic resonance imaging (MRI), which included coronal oblique T1-weighted and short tau inversion recovery (STIR) images of the SIJs, as well as assessments of the SpondyloArthritis Research Consortium of Canada index (SPARCC) and six consecutive coronal oblique layers [13].
-
7)
Quantitative Assessment of serum sclerostin level was performed using a Human Sclerostin enzyme immunoassay (ELISA) Kit (Bioassay Technology Laboratory, Zhejiang, China). Blood from the patient was drawn during the visit in three milliliters, centrifuged, and stored at -70 °C for analysis. The kit was completed for both patients and controls according to the manufacturer's instructions that were included in the kit.
Statistical analysis
The statistical software for social sciences, version 23.0 (SPSS Inc., Chicago, Illinois, USA), was used to analyze the recorded data. Mean ± standard deviation (SD) and ranges were used to show the quantitative data. Numbers and percentages were also used to represent qualitative characteristics. When comparing two means, the independent t-test of significance was employed.
The Chi-square test was used to compare groups based on qualitative data. The degree of correlation between two variables was evaluated using Pearson's correlation coefficient (r) test. Utilizing the Receiver Operating Characteristic (ROC) curve analysis, the overall predictivity of the parameter was determined, along with the optimal cut-off value. P-values less than 0.05 were considered significant, and P-values greater than 0.05 were insignificant.
Results
Twenty male AS patients, aged 25 to 45, with a mean age of 37.80 ± 7.02 and a mean disease duration of 6.4 ± 2.3 years, and twenty healthy age and sex-matched controls, aged 25 to 45, with a mean age of 37.75 ± 7.05, were included in this case–control study. All patients were on biological therapy. According to ASDAS, most patients had high disease activity, with a mean ± SD of 3.01 ± 0.81. Comparing ESR and CRP levels of the investigated cases to the control group, the AS patients showed substantial statistical differences (Table 1).
The mSASSS score of the spines in AS patients ranged from 2 to 19, With a mean ± SD of 8.40 ± 5.10. Five of the patients (25.0%) had Grade 1 sacroiliitis, 9 patients (45.0%) had Grade 2, and 6 patients (30.0%) had Grade 3 and patients were classified into mild, moderate, and severe, respectively. The mean ± SD of the SIJs SPARCC score was 17.60 ± 12.10; the spine SPARCC score was 16.74 ± 10.80; and the total SPARCC score was 34.34 ± 22.90 (Figs. 1 and 2).
Table 2 indicates a significant decrease in the serum concentrations of sclerostin in AS patients. Serum Sclerostin AS patients’ levels are represented by the ROC curve in Fig. 3. Higher sensitivity (100%) and specificity (100%) were observed for serum levels ≤ 20 ng/ml.
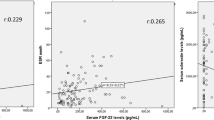
Table 3 summarizes the relationships between serum sclerostin levels and clinical, laboratory, and radiographic information AS patients. The BASMI score (p = 0.043), ESR, and CRP (p < 0.001) all exhibited a statistically significant negative correlation with serum sclerostin. No statistically significant correlation was found between serum sclerostin levels and each of BASDAI, BASFI, or ASDAS (p > 0.05). The level of serum sclerostin was found to be negatively correlated (p < 0.05) with the SIJ SPARCC score, spine SPARCC score, and total SPARCC score (Fig. 4). Despite this, there was no statistically significant link between the sacroiliac joint grading and spine radiographs, as indicated by Table 3. A substantially significant positive correlation was found between the mSASSS and BASMI (r = 0.409, p = 0.030).
Discussion
Understanding the pathophysiology of AS is essential to prevent bone formation, which is a major contributor to disability and a lower quality of life (QoL), particularly in cases where the disease is more active, the functional handicap is greater, the peripheral joints are more involved, and the spinal mobility is reduced [14].
We found the serum sclerostin level was considerably lower in male AS patients. The results of earlier research [15,16,17] are consistent with this observation.
In an earlier investigation, Appel et al. discovered that their AS patients had low serum and local bone tissue samples of sclerostin expression levels. This supports the theory of facilitated osteoblastic cell activation and differentiation by decreased Wnt inhibitor expression [2].
However, compared to the control group, Wakhulu et al. demonstrated that the AS patient group had a noticeably greater amount of serum sclerostin [18]. This could be explained by the fact that they did not include any patients getting biological treatment, which contrasted sharply with our results.
Moreover, in this work, serum sclerostin showed good diagnostic performance and was able to differentiate AS patients from controls with high sensitivity (100%) and specificity (100%) at a cut-off value ≤ 20 ng/ml.
Sclerostin levels were not shown to be correlated with either the BASDI or the ASDAS. Other research [2, 16, 19] reported similar outcomes. Saad et al. speculate that the low sample size and the possibility of additional cytokines or cellular mechanisms contributing to the downregulation of sclerostin expression could explain this finding [20]. However, we did not discover a meaningful association between serum sclerostin levels and the mSASSS. Previous research [15, 21] found results like these. Between the mSASSS and BASMI, we discovered a statistically significant positive correlation.
Inflammation and the production of new bone are correlated, according to Perotta et al. [15]. However, neither the existence of inflammation, the disease duration, nor the degree of disease activity affected sclerostin levels. Its role in the pathophysiology of AS is supported by the low serum levels of sclerostin in AS patients compared to controls.
Additionally, we found a statistically significant inverse relationship between the total SPARCC score, the spine SPARCC score, and the serum sclerostin’ level in SIJs. In line with Lau et al.'s findings [22], who discovered that neither the disease activity scores nor SPARCC showed a link. The lack of correlation may be attributed to the subjective nature of certain clinical disease activity scores, which rely on the experiences of individual patients. More research on a larger scale and with subgroup analysis could be required to assess this aspect.
Zhang et al. [23] showed a statistically significant correlation between clinical activity indices with SPARCC, which disagreed with our results. The fact that their sample size was greater (55 patients) might help to explain this.
The findings of our investigation point to a potential function for sclerostin in the diagnosis of AS patients. More research with large patient numbers is necessary to validate the role of sclerostin in disease activity and the condition's advancement.
Limitation of the study
-
1.
This cross-sectional case–control study prevents the establishment of causal relationships; so longitudinal studies and follow-up are necessary to confirm the correlation of Sclerostin to disease activity and disease progression.
-
2.
A small number of patient groups in the study; so, a wide scale of patients is needed to confirm the sensitivity and specificity of Sclerostin.
Conclusion
Serum sclerostin linked with MRI findings of disease activity, which may indicate subclinical activity status, and demonstrated good discriminating ability between cases of ankylosing spondylitis and healthy control subjects.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AS:
-
Ankylosing spondylitis
- ASAS:
-
Assessment of spondyloArthritis international society
- ASDAS:
-
Ankylosing spondylitis disease activity score
- BASDAI:
-
Bath AS disease activity
- BASMI:
-
Bath metrology index
- BASFI:
-
Bath ankylosing spondylitis functional index.
- mSASSS:
-
Modified stoke ankylosing spondylitis spinal score
- SPARCC:
-
SpondyloArthritis research consortium of Canada index
References
Magrey MN, Danve AS, Ermann J et al (2020) Recognizing axial spondyloarthritis: a guide for primary care. In Mayo Clinic Proceedings 95(11):2499–2508
Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R, Haibel H, Baraliakos X, Hempfing A, Rudwaleit M, Sieper J, Schett G (2009) Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 60(11):3257–3262. https://doi.org/10.1002/art.24888. (PMID: 19877044)
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S et al (2006) Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21(11):1738–1749
Burgers TA, Williams BO (2013) Regulation of Wnt/b-catenin signaling within and from osteocytes. Bone 54(2):244–249
Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J et al (2009) The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68(6):777–783
Jenkinson TR, Mallorie PA (1994) Whitelock HC Defining spinal mobility in ankylosing spondylitis (AS): the Bath AS Metrology Index. J Rheumatol 21:1694–1698
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291 (PMID: 7699630)
Lukas C, Landewé R, Sieper J et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68(1):18–24
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–5
Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 64:127–9
Van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 27(4):361–368
Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M, Conner-Spady B, Palsat J, Lambert RG (2005) Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 53(5):703–709. https://doi.org/10.1002/art.21445. (PMID: 16208659)
Sallam RA, Elbahnasawy AS (2020) Health-related quality of life (HRQoL) in ankylosing spondylitis patients: Relation to clinical features, disease activity, and radiographic damage. Egypt Rheumatol 42(4):287–290.https://doi.org/10.1016/j.ejr.2020.02.006. (ISSN 1110–1164)
Perrotta F, Ceccarelli F, Barbati C, Colasanti T, Socio De, Antonia & Scriffignano, Silvia & Alessandri, Cristiano & Lubrano, Ennio. (2018) Serum Sclerostin as a Possible Biomarker in Ankylosing Spondylitis: A Case-Control Study. J Immunol Res 2018:1–5. https://doi.org/10.1155/2018/9101964
Iaremenko O, Shynkaruk I, Fedkov D et al (2020) Bone turnover biomarkers, disease activity, and MRI changes of sacroiliac joints in patients with spondyloarthritis. Rheumatol Int 40(12):2057–2063
Klingberg E, Nurkkala M, Carlsten H et al (2014) Biomarkers of bone metabolism in ankylosing spondylitis about osteoproliferation and osteoporosis. J Rheumatol 41(7):1349–1356
Wakhlu A, Goel AP, Kumar P (2020) Prevalence of low bone mineral density in ankylosing spondylitis, correlation with disease activity, and serum sclerostin levels. Indian J Rheumatol 15:275–281
Taylan A, Sari I, Akinci B et al (2012) Biomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitis. BMC Musculoskelet Disord 13:191
Saad CG, Ribeiro AC, Moraes JC, Takayama L, Goncalves CR, Rodrigues MB, de Oliveira RM, Silva CA, Bonfa E, Pereira RM (2012) Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res Ther 14(5):R216. https://doi.org/10.1186/ar4055. (PMID: 23062122; PMCID: PMC3580528)
Sari I, Lee S, Tomlinson G et al (2021) Factors predictive of radiographic progression in ankylosing spondylitis. Arthritis Care Res 73(2):275–281
Lau HW, Mok CC, Chan WCS, Yuen MK, Li OC (2017) Intercorrelation between MRI disease activity scores of the sacroiliac joints and the spine, and clinical disease activity indices in patients with axial spondyloarthritis. Rep Med Imaging 10:45–51. https://doi.org/10.2147/RMI.S143393
Zhang P, Yu K, Guo R et al (2015) Ankylosing spondylitis: correlations between clinical and MRI indices of sacroiliitis activity. Clin Radiol 70:62–66
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
Nouran Medhat examined, analyzed, and interpreted the patient data regarding the disease. Marwa Ahmed was a major contributor to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent. The study was conducted by the World Medical Association Declaration of Helsinki for human patients and was approved by the ethics committee of the Faculty of Medicine Ain Shams University No. FMASU: MS 239/2022.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakrana, N.M.A.S., Badr, N.M.E., Hassan, M.A.A. et al. Serum sclerostin as a biomarker of disease activity in ankylosing spondylitis in correlation with radiographic imaging. Egypt Rheumatol Rehabil 51, 25 (2024). https://doi.org/10.1186/s43166-024-00258-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-024-00258-5