Abstract
The lack of valid biomarkers in patients with spondyloarthritis (SpA) requires searching for additional options to increase sacroiliac joint (SIJ) evaluation effectiveness. We assessed the serum levels of bone turnover markers and their relationships with active and chronic changes in SIJs using magnetic resonance imaging (MRI), indices, and laboratory parameters of disease activity in SpA patients. 102 patients with SpA and 15 healthy subjects were included. Testing of serum levels of transforming growth factor-beta (TGF-β1), Wnt3, sclerostin, and Dickkopf-1 (Dkk-1) was conducted. Active inflammatory lesions in SIJs were evaluated using Spondyloarthritis Research Consortium of Canada (SPARCC) MRI SIJ score, and chronic changes using the Danish scoring method. Bath Ankylosing Spondylitis Disease Activity Index, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), Ankylosing Spondylitis Disease Activity Scores with CRP, and ESR were used to assess disease activity. Serum levels of Dkk-1, TGF-β1, and sclerostin were significantly lower in SpA patients compared to healthy controls. The serum levels of Dkk-1 positively correlated with CRP. Dkk-1 had a significant negative correlation with Danish score. The sclerostin serum level had a weak negative correlation with the active inflammatory MRI SIJ lesions. There were positive correlations between TGF-β1 and sclerostin with Dkk-1, and negative correlation between Wnt3 and sclerostin. Dkk-1 positively correlated with CRP and negatively with chronic SIJ changes by Danish score. Sclerostin negatively correlated with the active SIJ lesions by SPARCC. This suggests that Dkk-1 and sclerostin are the most promising candidates to reveal inflammation and bone turnover in patients with SpA.
Similar content being viewed by others
References
Lukas C, Cyteval C, Dougados M, Weber U (2018) MRI for diagnosis of axial spondyloarthritis: major advance with critical limitations 'Not everything that glitters is gold (standard)'. RMD Open 4(1):e000586. https://doi.org/10.1136/rmdopen-2017-000586
Zhao Z, Wang G, Wang Y et al (2019) Correlation between magnetic resonance imaging (MRI) findings and the new bone formation factor Dkk-1 in patients with spondyloarthritis. Clin Rheumatol 38(2):465–475. https://doi.org/10.1007/s10067-018-4284-y
Maksymowych WP (2019) Biomarkers for diagnosis of axial spondyloarthritis, disease activity, prognosis, and prediction of response to therapy. Front Immunol 10:305. https://doi.org/10.3389/fimmu.2019.00305
Chiowchanwisawakit P, Lambert RG, Conner-Spady B, Maksymowych W (2011) Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum 63(8):2215–2225. https://doi.org/10.1002/art.30393
Maksymowych W, Chiowchanwisawakit P, Lambert R (2010) TNF blocking agents promote resolution of structural lesions in patients with spondyloarthritis. Ann Rheum Dis 69:262
Xie W, Zhou L, Li S, Hui T, Chen D (2016) Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Ann N Y Acad Sci 1364(1):25–31. https://doi.org/10.1111/nyas.12968
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42(4):606–615. https://doi.org/10.1016/j.bone.2007.12.224
Klingberg E, Nurkkala M, Carlsten H, Forsblad-d'Elia H (2014) Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol 41(7):1349–1356. https://doi.org/10.3899/jrheum.131199
Neve A, Corrado A, Cantatore FP (2012) Osteocytes: central conductors of bone biology in normal and pathological conditions. Acta Physiol 204(3):317–330. https://doi.org/10.1111/j.1748-1716.2011.02385.x
Morse A, McDonald MM, Kelly NH et al (2014) Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res 29(11):2456–2467. https://doi.org/10.1002/jbmr.2278
Diarra D, Stolina M, Polzer K et al (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163. https://doi.org/10.1038/nm1538
Santos A, Bakker AD, Willems HM et al (2011) Mechanical loading stimulates BMP7, but not BMP2, production by osteocytes. Calcif Tissue Int 89(4):318–326. https://doi.org/10.1007/s00223-011-9521-1
Appel H, Ruiz-Heiland G, Listing J et al (2009) Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 60(11):3257–3262. https://doi.org/10.1002/art.24888
Sieper J, Appel H, Rudwaleit M et al (2010) Inverse correlation between serum levels of dickkopf 1 (DKK 1), and new bone formation in ankylosing spondylitis patients. Ann Rheum Dis 69(3):442
Zhang L, Ouyang H, Xie Z, Liang ZH, Wu XW (2016) Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Exp Mol Med 48(4):e228. https://doi.org/10.1038/emm.2016.12
Kwon SR, Lim MJ, Suh CH et al (2012) Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol Int 32(8):2523–2527. https://doi.org/10.1007/s00296-011-1981-0
Ustun N, Tok F, Kalyoncu U et al (2014) Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reumatol Port 39(2):146–151
Perrotta FM, Ceccarelli F, Barbati C et al (2018) Serum sclerostin as a possible biomarker in ankylosing spondylitis: a case-control study. J Immunol Res 2018:9101964. https://doi.org/10.1155/2018/9101964
Rubio Vargas R, Melguizo Madrid E, González Rodríguez C et al (2017) Association between serum dickkopf-1 levels and disease duration in axial spondyloarthritis. Reumatol Clin 13(4):197–200. https://doi.org/10.1016/j.reuma.2016.04.013
Roberts AB, Sporn MB (1990) Peptide growth factors and their receptors. In: Handbook of experimental pharmakology. Springer-Verlag, New York
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61(4):337–346. https://doi.org/10.33549/physiolres.932289
Braun J, Bollow M, Neure L et al (1995) Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 38(4):499–505. https://doi.org/10.1002/art.1780380407
Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S (1999) Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281(18):1722–1727. https://doi.org/10.1001/jama.281.18.1722
Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD (2000) Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol 88:1192–1198. https://doi.org/10.1152/jappl.2000.88.4.1192
Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA (1994) Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest 93(2):892–899. https://doi.org/10.1172/JCI117045
Chen G, Deng C, Li YP (2012) TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8(2):272–288. https://doi.org/10.7150/ijbs.2929
Vaez F, Farazmand A, Shaaheen S et al (2017) Upregulation of transforming growth factor-B1 gene in ankylosing spondylitis patients. Rheum Res 2(3):103–107. https://doi.org/10.22631/rr.2017.69997.1026
Yang C, Ding P, Wang Q et al (2016) Inhibition of complement retards ankylosing spondylitis progression. Sci Rep 6:34643. https://doi.org/10.1038/srep34643
Howe HS, Cheung PL, Kong KO et al (2005) Transforming growth factor beta-1 and gene polymorphisms in oriental ankylosing spondylitis. Rheumatology 44(1):51–54. https://doi.org/10.1093/rheumatology/keh426]
Rudwaleit M, van der Heijde D, Landewé R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68(6):777–783. https://doi.org/10.1136/ard.2009.108233
Rudwaleit M, van der Heijde D, Landewé R et al (2011) The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70(1):25–31. https://doi.org/10.1136/ard.2010.133645
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291
Fernández-Espartero C, de Miguel E, Loza E et al (2014) Validity of the Ankylosing Spondylitis Disease Activity Score (ASDAS) in patients with early spondyloarthritis articlefrom the Esperanza programme. Ann Rheum Dis 73(7):1350–1355. https://doi.org/10.1136/annrheumdis-2012-202976
Maksymowych WP, Inman RD, Salonen D et al (2005) Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum 53(4):703–709. https://doi.org/10.1002/art.21337
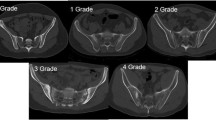
Madsen KB, Jurik AG (2010) Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res 62(1):11–18. https://doi.org/10.1002/acr.20008
Daoussis D, Liossis SNC, Solomou EE et al (2010) Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum 62(1):150–158. https://doi.org/10.1002/art.27231
Akinci B, Bayraktar F, Saklamaz A et al (2007) Low transforming growth factor-β1 serum levels in idiopathic male osteoporosis. J Endocrinol Invest 30:350–355. https://doi.org/10.1007/BF03346309
Perpétuo IP, Caetano-Lopes J, Vieira-Sousa E et al (2017) Ankylosing spondylitis patients have impaired osteoclast gene expression in circulating osteoclast precursors. Front Med (Lausanne) 4:5. https://doi.org/10.3389/fmed.2017.00005
Claudepierre P, Rymer JC, Authier FJ et al (2017) A relationship between TGF-beta 1 or IL-6 plasm levels and clinical features of ankylosing spondylitis. Br J Rheumatol 36(3):400–401. https://doi.org/10.1093/rheumatology/36.3.400
van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE (2014) Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab 99(2):E252–E256. https://doi.org/10.1210/jc.2013-3278
Molto A, Gossec L, Lefèvre-Colau M-M et al (2019) Evaluation of the performances of ‘typical’ imaging abnormalities of axial spondyloarthritis: results of the cross-sectional ILOSDESIR study. RMD Open 5(1):e000918. https://doi.org/10.1136/rmdopen-2019-000918
Weber U, Zubler V, Zhao Z et al (2015) Does spinal MRI add incremental diagnostic value to MRI of the sacroiliac joints alone in patients with non-radiographic axial spondyloarthritis? Ann Rheum Dis 74(6):985–992
Funding
We did not have any financial support.
Author information
Authors and Affiliations
Contributions
All authors participated in the analysis and interpretation of the data, reviewed and provided feedback on the draft manuscript, and made the decision to submit the manuscript for publication. All authors vouch for the completeness and accuracy of the data and analyses. Material preparation, literature search, and data analysis were performed by OI, IS, DF, and LP. Laboratory methods and specimen handling for biomarkers were performed by KI. The first draft of the manuscript was written by DF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Oleg Iaremenko, Iuliia Shynkaruk, Dmytro Fedkov, Kateryna Iaremenko, and Liubov Petelytska declare that they have no conflict of interest.
Ethical approval
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and was approved by the appropriate institutional review boards (Local Ethic Committee of Bogomolets National Medical University, protocol #58, 26 Feb 2016). Authors fulfilled the ICMJE authorship criteria.
Informed consent
Written informed consent was obtained from all patients prior to study start.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iaremenko, O., Shynkaruk, I., Fedkov, D. et al. Bone turnover biomarkers, disease activity, and MRI changes of sacroiliac joints in patients with spondyloarthritis. Rheumatol Int 40, 2057–2063 (2020). https://doi.org/10.1007/s00296-020-04708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04708-z