Abstract
Background
Acute hyperglycemia is a common finding in both diabetic and non-diabetic patients with acute coronary syndrome (ACS) who present to the emergency department (ED). The prognostic role of hyperglycemia in diabetic patients with ACS remains controversial at least on the short-term basis. The aim of the present study was to find the relation between the glycemic gap and clinical outcome in diabetic patients with ACS.
Methods
The study included 100 diabetic patients with ACS to who were prospectively followed during their hospital stay. Admission blood glucose was measured and glycemic gap was calculated.
Results
In diabetic patients suffering ACS, there was a significant relation between the glycemic gap value, occurrence of major adverse cardiovascular events (MACE) and length of hospital stay.
Conclusion
Glycemic gap is a better marker than admission blood glucose alone in diabetic patients presenting with ACS. This study added the optimal cut-off value for this important biomarker.
Similar content being viewed by others
Background
Acute hyperglycemia is a common finding in patients who present to the emergency department (ED) suffering acute coronary syndrome (ACS) in both diabetic and non-diabetic patients. The prognostic role of hyperglycemia in non-diabetic patients with (ACS) may be well established, compared to diabetic patients in whom it remains controversial at least on the short-term basis [1, 2]. In diabetic patients, hyperglycemia is the cardinal feature which may be noticed regardless of a stressful event due to many causes as poor glycemic control [3].
The chronic effects of hyperglycemia are associated with long-term dysfunction, damage, and failure of various organs, especially the nerves, kidneys, eyes, heart, and blood vessels [4].
Stress hyperglycemia is defined as a transient increase in blood glucose concentration during acute illness. It represents two distinct populations of patients; those with undiagnosed diabetes or impaired glucose tolerance, and those who develop hyperglycemia as the result of hormonal surges in response to severe stress. Evidence shows that stress hyperglycemia involves increased insulin resistance in tissues and organs, increased gluconeogenesis, decreased glycogenolysis, and increased lipolysis. Elevated oxidative stress and increased serum levels of pro-inflammatory cytokines, cortisol, and glucagon promote these activities [5,6,7].
In patients with ACS, the concomitant occurrence of hyperglycemia enhances the risk of morbidity and mortality whether or not the patient had a prior diagnosis of diabetes. Stress hyperglycemia shares many properties with hyperglycemia associated with type 2 diabetes, including increased oxidative stress, inflammation, and activation of stress-responsive kinases. Infarcts are usually larger in patients with stress-related hyperglycemia. Severe infarction and increased sensitivity to ischemia-reperfusion injury are predictors of poor prognosis in ACS patients with stress hyperglycemia. Evidence from clinical and preclinical studies suggests that insulin resistance and glucose homeostasis play key roles by predisposing hyperglycemic myocardial tissue to injury during ischemia and reperfusion [8, 9].
Methods
Study population
In this study, a total of 113 diabetic patients aged between 40 and 81 years were recruited from emergency department at Alexandria Main University Hospital, Egypt.
Thirteen patients were excluded for acute coronary syndrome mimics, (seven diagnosed as pneumonia, three diagnosed as pericarditis, two diagnosed as pneumothorax, and one diagnosed as aortic dissection). Statistics were done on 100 patients who met the inclusion criteria.
Patients younger than 18 years, pregnant females, patients presented with hemodynamic instability, and those with hemoglobinopathies were excluded from this study. All the participants included in the study were informed about the nature of the study, and their consent on participating voluntarily was obtained. The study was approved by the Ethical Committee of the Faculty of Medicine, Alexandria University.
After giving their consent, all study participants were subjected to full medical history assessment including demographic details, characters and duration of the chest pain, risk factors for ACS, and complete physical examination was done for all patients.
Biochemical analysis
Blood samples were collected from all patients for blood gases analysis and cardiac biomarkers of ischemia, i.e., CK-MB and high sensitivity Troponin. Glycated hemoglobin (HbA1c) percentage was determined using high performance liquid chromatography (HPLC) method. Random blood glucose was measured by finger stick sample for capillary blood glucose measurement which was subsequently confirmed by serum blood glucose level in the laboratory.
A 12-lead ECG was done for all patients within 10 min of presentation and all patients had a chest radiograph to exclude the risk of ACS mimics, e.g., aortic dissection, pneumothorax, and pneumonia.
The following formula was used to convert HbA1c levels to the estimated A1c-Derived Average glucose (ADAG) levels: 28.7 × HbA1c − 46.7 [10]. The glycemic gap, which shows changes in blood glucose levels during the acute event, was calculated from the glucose level measured at ED minus the ADAG level [11].
The glycemic gap had been correlated with the patients’ outcome which was assessed by the complications that occurred to the patient during his duration of stay and the length of that stay. Complications during hospitalization were considered in any patient that witnessed a sudden cardiac arrest, life threatening arrhythmias or acute pulmonary edema [12,13,14].
Statistical analysis
Collected data were expressed as mean and standard deviation and were analyzed using the two-tailed Student’s t test. Categorical data are expressed as frequencies (%) and were evaluated using the chi-square test or Fisher’s exact test. A one-way analysis of variance was used to assess the significance of various characteristics, laboratory data, and adverse outcomes. A post-hoc analysis was performed using the Bonferroni test. A receiver–operator characteristic curve (ROC) curve was plotted to analyze the discriminative power of the prediction tools, and the area under the ROC (AUROC) and the corresponding 95% confidence intervals (CI) were calculated.
Univariate and multivariate Cox hazard regression analyses were performed to identify the risk factors associated with MACEs. Variables with a p < 0.05 in the univariate analysis were entered into the multivariate Cox hazard regression analysis. The correlation between glycemic gap and continuous variables was evaluated by the Pearson product-moment correlation. The correlation between the glycemic gap and ordinal variables was evaluated by the Spearman’s rank-order correlation. The data were analyzed using Statistical Package for the Social Sciences statistical software (SPSS, Inc., Chicago, IL, USA), version 20.0 and differences with p values < 0.05 were considered statistically significant.
Results
This study was conducted on 100 diabetic patients who presented to ED with ACS. The study included 74 males and 26 females with a mean age of 60.33 ± 10.06 years.
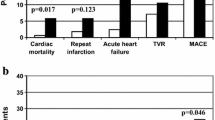
Thirty six patients witnessed major adverse cardiac event (MACE) during their hospital stay where 6 patients suffered cardiac arrest, 24 patients suffered pulmonary edema, and 13 patients suffered life threatening dysrhythmias; of note, some patients had more than one complication during their hospital stay.
Diabetic patients who suffered MACE had significantly higher glycemic gap compared to patients who did not have MACE (p < 0.001) while there was no significant difference between both groups regarding admission random blood glucose (RBG) where p = 0.879 as shown in Table 1.
There was a significant positive correlation the glycemic gap value and the occurrence of MACE (Fig. 1), there was a significant negative correlation between HbA1c level and the occurrence of MACE in diabetic patients suffering ACS (Fig. 2).
Moreover, on performing regression analysis, out of all criteria, there was significant relation between age and MACE occurrence with a p value < 0.001. The Cox proportional hazard model revealed that the hazard ratio of the glycemic gap (mg/dL) for MACE was 1.028 (95% CI 1.000–1.005, p < 0.001). Therefore it was clear that the glycemic gap and age are independent predictors of MACE occurrence in diabetic patients with ACS. The results of univariate and multivariate analyses are shown in Table 2.
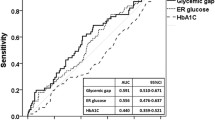
The ROC curve analysis for the glycemic gap value to predict MACE in diabetic patients suffering ACS, it was found that the optimal cut-off value of the glycemic gap was 55 mg/dl with maximum AUROC of 0.796 (95% CI = 0.702–0.891) (sensitivity 86.11% and specificity 56.25%). Moreover, glycemic gap was more specific rather than admission RBG level in predicting MACE as shown in Fig. 3 and Table 3.
Moreover, a significant positive correlation was found between glycemic gap value and the length of hospital stay of ACS patients with diabetes; either coronary care unit (CCU), ward stay, or both collectively with p value < 0.001. There was also a statistical inverse significant correlation between HbA1c level and the total hospital stay (p = 0.036) but there was no statistical significance between admission RBG and the length of hospital stay (p = 0.064)
Discussion
Cardiac arrest, pulmonary edema and life-threatening dysrhythmia are fatal complications that may occur following ACS. Early identification of patients who are at high risk of developing those complications may help in reducing morbidity and mortality [15]. Numerous studies have shown that hyperglycemia is a commonly encountered issue in critically ill patients in ED and in the critical care settings even in patients without diabetes mellitus [16,17,18,19,20]. A recent analysis of medical records showed that hyperglycemia was present in 38% of adult patients admitted to hospital, of whom 26% had a known history of diabetes, and 12% had no history of diabetes before the admission [19].
In this context, the adverse prognostic impact of hyperglycemia which accompanies ACS was paid considerable attention of the medical societies. It is now well established that acute hyperglycemia that accompanies ACS at presentation is one of the predictors of poor outcomes upon hospital admission and an important prognostic marker for all-cause death in patients with ACS, whether or not they had previously known diabetes mellitus [5, 21, 22].
Although many studies were conducted on the effect of admission hyperglycemia on the short- and long-term outcome of the acute coronary syndrome (ACS) [2, 23,24,25] unfortunately, there is too scarce available literature about the impact of the glycemic gap on ACS outcome [11].
The main finding in the present prospective study was that the glycemic gap is strongly and significantly related to the occurrence of MACE (p value < 0.001) and longer hospital stay duration (p value < 0.001) in diabetic patients presented to the ED with ACS. These results mimic a retrospective observational study which was conducted by Liao et al. (2016) who enrolled 331 patients to their study, of these patients, 43 (13.0%) died during hospitalization and 61 (18.4%) experienced MACEs and found a relation between elevated glycemic gap and adverse outcomes in diabetic patients presented with acute myocardial infarction. Compared with survivors, non-survivors had a statistically significant higher glycemic gap and longer hospital stay [11].
Reviewing previous literature revealed a lot of studies their authors were concerned about finding an association between hyperglycemia and ACS outcome. Capes et al. (2000) found that acute hyperglycemia with myocardial infarction was associated with an increased risk of in-hospital mortality in patients with and without diabetes with increased risk of congestive heart failure or cardiogenic shock in patients without diabetes [5].
K. Foo et al. (2003) studied the relation between a single admission blood glucose value and ACS outcome. They found a marked correlation between hyperglycemia and ACS outcomes. They also found that prognostic correlates of admission glycemia were applied equally to diabetic and non-diabetic subgroups as in both subgroups, the more the hyperglycemia the more the risk of heart failure and cardiac arrest [26]. Sousa et al. (2013) have observed that admission hyperglycemia is an independent predictive factor for in-hospital complications after ACS in diabetic and non-diabetic patients [27].
The risk of acute hyperglycemia was studied by Angeli et al. (2013), and they found that acute hyperglycemia documented during ACS brings an excess risk of mortality not only in the hospital setting [28], but also in the short term (30 days) and long term (up to 108 months). Jacob Lønborg et al. (2014) studied the impact of acute hyperglycemia on myocardial infarct size in patients with STEMI. They concluded that hyperglycemia could serve as a marker for the severity of myocardium at risk and injury [29]. All those studies gave a well-established proof about the association between acute hyperglycemia and adverse outcome after ACS, but no studies highlighted the role and cut-off value of glycemic gap as the current study.
Another point of interest was that many of these studies found no difference regarding the outcome between diabetics and non-diabetics. Some of them even found poor prognosis in non-diabetic patients. This “diabetes paradox” had been continuously observed in other studies with no explanation for that finding except for that there was a hidden factor that was only applied on diabetic patients and not on non-diabetics [3, 30].
Recently, many studies have been concerned about using the glycemic gap in diabetic patients as a predictor for poor outcome in many aspects, e.g., ICU outcomes, community-acquired pneumonia, necrotizing fasciitis, acute heart failure, and acute coronary syndrome. All of them related the glycemic gap to adverse outcome and so more hospital stay length but without determining the glycemic gap cut-off value [31,32,33,34,35].
Generally, the stress response is known as an adaptive process for survival which benefits in drastically disturbed physiological situations, such as acute illness. Stress triggers systemic inflammatory response that accompanies secondary complications, such as acute hyperglycemia and insulin resistance. Stress-induced hyperglycemia has a direct correlation with the morbidity and mortality rates in critical illness.
Stress hyperglycemia is the result of sympathetic nervous system activation and the hypothalamic–pituitary axis with subsequent increased production of catecholamine and cortisol levels that stimulate gluconeogenesis, glycogenolysis, and lipolysis. Surprisingly, morbidity and mortality associated with hyperglycemia were especially severe in patients who were not previously diagnosed as diabetics. In diabetic patient with ACS, stress-induced hyperglycemia represents the fraction of hyperglycemia that represents the damage limit on the level of myocardium. Stress-induced hyperglycemia had been reported to be associated with acute adrenergic signal of stress and endothelial cell dysfunction in acute myocardial infarction, which was partially attributed to endothelial cell apoptosis, reactive oxygen species (ROS) over production and inflammation [36,37,38].
Most of studies which were concerned about the relation between HbA1c and ACS outcomes that were conducted in the past, whether found no relation between them [39,40,41] or found a relation but on the long term [42, 43]. Very few studies found a relation between glycosylated hemoglobin and ACS short-term outcomes.
In the current study, it was found that HbA1c measured values were inversely related to MACE occurrence and long hospital stay with a statistically significant relation. Results which were discordant with most of the previously conducted studies except one study conducted by Li et al. (2014), who reported that higher levels of HbA1c were associated with less risk of myocardial injury following PCI in diabetic patients because of better energy supply. As a large number of ACS patients included in the current study had primary PCI (n = 57), this might account for the confounding factor in short-term prognosis [44].
Limitations
A number of limitations have influenced the current study. The sample size was relatively small, the study was hospital-based where all participants were of the same ethnicity, and therefore the findings may be not generally applicable to other population. Also, glycemic control during hospitalization was not implied for and correlated to the results.
The study highlighted the complications that occurred during the hospital stay only, which provided us with a short-term follow-up for patients as far as a maximum of 2 weeks. This creates a limitation to the study in giving information about the long-term outcomes.
Case study
A 61-year-old diabetic male patient with history of CCS and smoking with STE-ACS managed by primary PCI. His admission blood glucose is 331 mg/dl, his HbA1c is 8.2 and his glycemic gap measurement is 142 mg/dl. This patient spent 16 days in the hospital (3 ward days and 13 CCU days). During which he witnessed pulmonary edema, ventricular fibrillation and cardiac arrest once.
A 58-year-old diabetic male patient with history of CCS and smoking with STE-ACS managed by primary PCI. His admission blood glucose is 352 mg/dl, his HbA1c is 11.95 and his glycemic gap measurement is 55 mg/dl. This patient’s hospital stay was 4 days (2 CCU days and 2 ward days). He did not witness any complications.
-
The two patients have the same age, the same risk factors, the same diagnosis, and the same line of management but they have followed two different outcomes.
-
Comparing the RBG in the two cases reveals that the case that witnessed MACE had the lower RBG value of RBG in comparison with case 2.
-
Comparing the glycemic gap value in the two cases reveals that the case that witnessed MACE had a higher glycemic gap value in comparison with case 2.
Conclusions
Although many studies stated that glycemic gap could be used as a biomarker for predicting MACE and duration of hospital length in diabetic patients with ACS, and that it is a better marker than admission blood glucose alone in diabetic patient presented with ACS, but our study added the optimal cut-off value of this important biomarker which is a glycemic gap of 55 mg/dl, to predict complications.
Availability of data and materials
All related data and materials are available on proper request.
References
Norhammar A, Tenerz Å, Nilsson G, Hamsten A, Efendíc S, Rydén L et al (2002) Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359(9324):2140–2144
Shah B, Amoroso NS (2012) Sedlis SPJTAjotms. Hyperglycemia in nondiabetic patients presenting with acute myocardial infarction. Am J Med Sci 343(4):321–326
Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C et al (2008) Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med 36(8):2249–2255
American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes care 37(Supplement 1):S81–90
Capes SE, Hunt D, Malmberg K, Gerstein HCJTL (2000) Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Crit Care Med 355(9206):773–778
Fava S, Aquilina O, Azzopardi J, Muscat HA, Fenech FFJDm. (1996) The prognostic value of blood glucose in diabetic patients with acute myocardial infarction. J Am College Cardiol 13(1):80–83
Malmberg K, Rydén L, Hamsten A, Herlitz J, Waldenström A, Wedel HJCr. (1997) Mortality prediction in diabetic patients with myocardial infarction: experiences from the DIGAMI study. Euopean Soc Cardiol 34(1):248–253
Esposito K, Marfella R, Giugliano DJDc. (2003) Stress hyperglycemia, inflammation, and cardiovascular events. Am Diabetes Assoc 26(5):1650–1651
Marfella R, Siniscalchi M, Esposito K, Sellitto A, De Fanis U, Romano C et al (2003) Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Am Diabetes Assoc 26(11):3129–3135
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJJDc. (2008) Translating the A1C assay into estimated average glucose values. New Engl J Med 31(8):1473–1478
Liao W-I, Lin C-S, Lee C-H, Wu Y-C, Chang W-C, Hsu C-W et al (2016) An elevated glycemic gap is associated with adverse outcomes in diabetic patients with acute myocardial infarction. Scientific Rep J 6(1):1–11
Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M et al (2004) ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). AHA 44(3):671–719
Komajda M, Follath F, Ko S, Cleland J, Aguilar J, Cohen-Solal A et al (2003) The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 2: treatment. AHA 24(5):464–474
Marijon E, Uy-Evanado A, Dumas F, Karam N, Reinier K, Teodorescu C et al (2016) Warning symptoms are associated with survival from sudden cardiac arrest. Ann Int Med 164(1):23–29
Kumar A, Cannon CP (2009) Acute coronary syndromes: diagnosis and management, part I. InMayo Clinic Proceedings (Vol. 84, No. 10). Elsevier, p. 917–38
Bartnik M, Ryden L, Ferrari R, Malmberg K, Pyörälä K, Simoons M et al (2004) The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: The Euro Heart Survey on diabetes and the heart. Eur Heart J 25(21):1880–1890
Finney SJ, Zekveld C, Elia A, Evans TWJJ (2003) Glucose control and mortality in critically ill patients. JAMA network 290(15):2041–2047
Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C et al (2012) Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med J 40(12):3251–3276
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE et al (2002) Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87(3):978–982
Viana MV, Moraes RB, Fabbrin AR, Santos MF, Gerchman FJRBdti. (2014) Assessment and treatment of hyperglycemia in critically ill patients. Revista Brasiliera De Terapia Intensive 26(1):71–76
Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T et al (2008) Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. AHA 117(12):1610–1619
Guinot D, De Angeli A, Garassino A (2010) Holthuisea, a new genus from the Eocene of Italy (Decapoda, Brachyura, Hexapodidae). InStudies on Malacostraca: Lipke Bijdeley Holthuis Memorial, Volume Volume 2010 Jan 1. Brill, pp. 283–304
Dandona P, Chaudhuri AJNRE (2014) Diabetes: glycaemia and insulin after acute myocardial infarction. Lancet J 10(8):448
Deckers JW, van Domburg RT, Akkerhuis M, Nauta STJTAjoc. (2013) Relation of admission glucose levels, short-and long-term (20-year) mortality after acute myocardial infarction. Am J Cardiol 112(9):1306–1310
Stranders I, Diamant M, van Gelder RE, Spruijt HJ, Twisk JW, Heine RJ et al (2004) Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Jama Int Med 164(9):982–988
Foo K, Cooper J, Deaner A, Knight C, Suliman A, Ranjadayalan K et al (2003) A single serum glucose measurement predicts adverse outcomes across the whole range of acute coronary syndromes. BMJ J 89(5):512–516
Pinheiro CP, Oliveira MD, Faro GB, Silva EC, Rocha EA, Barreto-Filho JA, Oliveira JL, Sousa AC (2013) Prognostic value of stress hyperglycemia for in-hospital outcome in acute coronary artery disease. Arquivos brasileiros de cardiologia 100:127–34
Angeli F, Reboldi G, Poltronieri C, Verdecchia P (2013) Hyperglycemia during acute coronary syndrome: prognostic implications. J Diabetes Metab 4(7)
Lønborg J, Vejlstrup N, Kelbæk H, Nepper-Christensen L, Jørgensen E, Helqvist S et al (2014) Impact of acute hyperglycemia on myocardial infarct size, area at risk, and salvage in patients with STEMI and the association with exenatide treatment: results from a randomized study. Am Diabetes Assoc 63(7):2474–2485
Cao JJ, Hudson M, Jankowski M, Whitehouse F, Weaver WDJTAjoc. (2005) Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Sci Rep J 96(2):183–186
Chen PC, Liao WI, Wang YC, Chang WC, Hsu CW, Chen YH, Tsai SH (2015) An elevated glycemic gap is associated with adverse outcomes in diabetic patients with community-acquired pneumonia. Medicine 94(34)
Donagaon S, Dharmalingam MJIjoe, metabolism. (2018) Association between Glycemic Gap and adverse outcomes in critically ill patients with diabetes. Indian J Endocrinol Metab 22(2):208
Fawzy F, Saad MS, AM ES, MMJD E (2019) Research MSC, Reviews. Effect of glycemic gap on short term outcome in critically ill patient: In zagazig university hospitals. Sci Direct J 13(2):1325–1328
Liao WI, Wang JC, Chang WC, Hsu CW, Chu CM, Tsai SH (2015) Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine 94(36).
Liao W-I, Wang J-C, Lin C-S, Yang C-J, Hsu C-C, Chu S-J et al (2019) Elevated glycemic gap predicts acute respiratory failure and in-hospital mortality in acute heart failure patients with diabetes. Sci Rep J 9(1):1–9
Hyseni A, Roest M, Braun SL, Barendrecht AD, de Groot PG, Ndrepepa G et al (2013) Chronic dysfunction of the endothelium is associated with mortality in acute coronary syndrome patients. Thrombosis Res J 131(3):198–203
Su G, Mi SH, Li Z, Tao H, Yang HX, HJCd Z (2013) Prognostic value of early in-hospital glycemic excursion in elderly patients with acute myocardial infarction. Cardiovasc Diabetol J 12(1):33
Ye Y, Xie H, Zhao X, Zhang SJC (2012) The oral glucose tolerance test for the diagnosis of diabetes mellitus in patients during acute coronary syndrome hospitalization: a meta-analysis of diagnostic test accuracy. Cardiolovasc Diabetol 11(1):155
Geng J, Zhang Y, Wang B, Xie J, Xu B, Li J (2017) Glycosylated hemoglobin levels and clinical outcomes in nondiabetic patients with coronary artery disease: A meta-analysis. Medicine 96(17)
Kowalczyk J, Mazurek M, Zielinska T, Lenarczyk R, Sedkowska A, Swiatkowski A et al (2015) Prognostic significance of HbA1c in patients with AMI treated invasively and newly detected glucose abnormalities. SAGE Journal 22(6):798–806
Savonitto S, Morici N, Nozza A, Cosentino F, Perrone Filardi P, Murena E et al (2018) Predictors of mortality in hospital survivors with type 2 diabetes mellitus and acute coronary syndromes. SAGE Journal 15(1):14–23
Østergaard H, Mandrup-Poulsen T, Berkelmans G, van der Graaf Y, Visseren F, Westerink J et al (2019) Limited benefit of haemoglobin glycation index as risk factor for cardiovascular disease in type 2 diabetes patients. Cardiolovasc Diabetol 45(3):254–260
She J, Deng Y, Wu Y, Xia Y, Li H, Liang X et al (2017) Hemoglobin A 1c is associated with severity of coronary artery stenosis but not with long term clinical outcomes in diabetic and nondiabetic patients with acute myocardial infarction undergoing primary angioplasty. Cardiolovasc Diabetol 16(1):97
Li XL, Li JJ, Guo YL, Zhu CG, Xu RX, Li S, Qing P, Wu NQ, Jiang LX, Xu B, Gao RL (2014) Relationship of glycated hemoglobin levels with myocardial injury following elective percutaneous coronary intervention in patients with type 2 diabetes mellitus. PLoS One 9(7):e101719
Acknowledgements
This work was supported by Internal Medicine Department (Diabetes Unit), Faculty of Medicine,
Alexandria University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors preformed the study equally, contributed to the extraction of data, analyzed the data, wrote the paper, and approved the manuscript. All authors have critically reviewed and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of the Faculty of Medicine, Alexandria University, and a written consent for participation in the study was obtained from all included subjects. Date of approval: 16 May 2019. Serial no.: 0105999. IRB No.: 00012098. FWA No.: 00018699.
Consent for publication
All patients participating in study signed an informed consent according to our intuitional regulation and were submitted to our institutional ethical committee.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghanem, Y.M., Ayad, M.W., Kareem, A.A. et al. Glycemic gap and the outcome of diabetic patients presenting with acute coronary syndrome. Egypt J Intern Med 34, 10 (2022). https://doi.org/10.1186/s43162-022-00099-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00099-8