Abstract
Background
The appropriateness of the routine performance of an oral glucose tolerance test (OGTT) to screen for diabetes mellitus (DM) during acute coronary syndrome hospitalization is still under debate.
Methods
A systematic search of databases (MEDLINE [1985 to March 2012], EMBASE [1985 to March 2012]) was conducted. All prospective cohort studies assessing the accuracy or reproducibility of an OGTT in ACS or non-ACS individuals were included. A bivariate model was used to calculate the pooled sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). Heterogeneity was explored using subgroup analysis and meta-regression.
Results
Fifteen studies with 8,027 participants were included (10 ACS and 5 non-ACS studies). The pooled results on SEN, SPE, PLR, NLR, and DOR were 0.70 (95% CI, 0.60-0.78), 0.91 (95% CI, 0.86-0.94), 7.6 (95% CI, 4.9-11.7), 0.33 (95% CI, 0.25-0.45), and 23 (95% CI, 12–41), respectively. The OGTT has a slightly lower SPE in diagnosing DM in ACS than in non-ACS patients (0.86 [95% CI 0.81-0.92] versus 0.95 [95% CI 0.93-0.98], p<0.01), while the SEN values are comparable (0.71 [95% CI 0.60-0.82] versus 0.67 [95% CI 0.54-0.81], p=0.43). After adjusting the interval between repeated tests and age, the meta-regression did not show a difference in DOR between ACS and non-ACS studies.
Conclusions
Despite the discrepancy in the interval between the two OGTTs, performing an OGTT in patients with ACS provides accuracy that is similar to that in in non-ACS patients. It is reasonable to screen patients hospitalized for ACS for previously undiagnosed DM using an OGTT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Numerous studies have demonstrated that hyperglycemia is common among patients with acute coronary syndrome (ACS)[1, 2], and the relationship between hyperglycemia and increased mortality risk in ACS has been well established across various glucose metrics[3, 4].
However, considering its accuracy and reproducibility in stress condition, the routine performance of an oral glucose tolerance test (OGTT) to diagnose diabetes during the acute phase of ACS is still the subject of ongoing debate. The European guidelines on diabetes, pre-diabetes, and cardiovascular diseases recommend the performance of an OGTT in patients with established cardiovascular disease[5]. Furthermore, the European guidelines on the management of acute myocardial infarction in patients presenting with persistent ST-segment elevation specify that an OGTT should be performed before or shortly after hospital discharge[6]. In regards to the management of hyperglycemia in ACS, the NICE (National Institute for Health and Clinical Excellence) does not recommend the routine use of the OGTT in patients with hyperglycemia after ACS without known diabetes if hemoglobin A1C and fasting blood glucose levels are within the normal range[7]. A scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism does not encourage routine use of the OGTT for screening during the hospital stay[8].
Thus, this meta-analysis of prospective cohort studies to determine the accuracy of the OGTT in the diagnosis of diabetes in the acute phase of ACS compared to that in non-ACS cases was conducted to clarify this dispute with evidence.
Methods
A protocol was designed that detailed the objective of our analysis, the criteria for study inclusion/exclusion, the assessment of study quality, the primary outcome, and the statistical methods in accordance with the MOOSE guideline for meta-analysis of observational studies[9].
Data sources and searches
A search of MEDLINE (1985 to March 2012) and EMBASE (1985 to March 2012) via EMBASE.com was conducted to identify all studies involving diagnostic tests assessing the value of the OGTT in the diagnosis of diabetes mellitus in subjects with or without ACS (Additional file1) (Yicong Ye and Hongzhi Xie). In addition, a manual search of the literature using the references of original manuscripts, reviews, and meta-analyses was performed. Finally, a search of the Cochrane database of ongoing systemic reviews was conducted. No language restriction was imposed.
Study selection
The study eligibility was independently determined by two reviewers (Yicong Ye and Hongzhi Xie). Disagreement was resolved by consensus. The study eligibility criteria included: 1) published prospective cohort studies, 2) performing the first OGTT during ACS hospitalization (only for ACS studies), 3) repeating an OGTT more than 1 week and less than 3 years after the first one, and 4) use of a 75 g OGTT.
The following were criteria for exclusion: 1) pregnant and pediatric individuals, 2) patients with other chronic diseases, other than coronary heart disease, such as cystic fibrosis, polycystic ovary syndrome, acromegaly, liver disease, and renal disease, 3) studies in which the OGTT was repeated only in subjects with abnormal or normal OGTT results in the first test, and 4) studies with intended medical intervention (life-style change or medication) between administration of the two OGTTs.
Attempts were made to contact the author for further information on studies that fulfilled the above criteria but did not have sufficient data to build a two-by-two table before they were excluded from the final analysis.
Data extraction and quality assessment
Data extraction was carried out independently by two authors (Yicong Ye and Hongzhi Xie). Disagreements were resolved by discussion between the two reviewing authors. From each included trial, information was extracted on: 1) the study population, 2) the published language, 3) the time interval between administration of the two OGTTs, 4) the reference standard of diabetes, 5) mean age of study population and 6) the true positive value, false positive value, false negative value, and true negative value of each included study.
Study quality was assessed using the QUADAS list, with each item scored as “yes”, “no”, or “unclear”[10]. The results are presented in the text and in a graph. A summary score was not calculated when estimating the overall quality of an article since the interpretation of such summary scores is problematic and potentially misleading. The items in the QUADAS tool and their interpretation are presented in Additional file1.
Data synthesis and analysis
The WHO (World Health Organization) 1985[11], ADA (American Diabetes Association) 2003[12], or WHO 1999[13] criteria was used as the reference standard depending on which criteria had been used in each study. All the included patients can be classified into two groups: diabetes and non- diabetes. The diagnostic threshold was the same as the reference standard in each included study. Accordingly, the two-by-two tables were constructed. The data in the two-by-two tables were used to calculate the sensitivity and specificity for each study. The individual study results were presented graphically by plotting the estimates of sensitivity (SEN) and specificity (SPE), and their 95% confidence intervals (95% CI), in paired forest plots.
A bivariate model was used for the meta-analysis of the pairs of SEN and SPE and for the construction of a summary receiver operating characteristic (ROC) curve[14]. This summary ROC curve represents the change in diagnostic accuracy according to changes in the cutoff value. The bivariate random effects approach enabled the calculation of summary estimates of SEN, SPE, the positive likelihood ratio (PLR), the negative likelihood ratio (NLR), and the diagnostic odds ratio (DOR) while correctly dealing with the different sources of variation: (1) the imprecision by which SEN and SPE were measured within each study, (2) variation beyond chance in SEN and SPE between studies, and (3) any correlation that might exist between SEN and SPE.
The heterogeneity (or absence of homogeneity) of the results between studies was assessed statistically using the quantity I2, which describes the percentage of total variation across studies that is attributable to heterogeneity rather than chance[15]. Covariates were incorporated in the bivariate model to examine the effect of potential sources of heterogeneity across subgroups of studies. Due to the bivariate nature of the model, the effects of covariates on SEN and SPE can be modeled separately. The following covariates were analyzed to explore the heterogeneity: 1) Study population: the major objective of the studies was to compare the diagnostic accuracy in patients with and without ACS; 2) Threshold: although different criteria were used in the studies (including WHO 1985, ADA 2003, and WHO 1999), all studies used the same cutoff value of a 2-hour OGTT (11.1mmol/L) to diagnose diabetes. The major difference is that some of the studies included FBG in the diagnosis criteria, while others did not. Thus, the studies were divided into 2 subgroups (with or without FBG). 3) Blood sample: different reproducibility values for plasma and capillary glucose have been reported. 4) Interval between repeated tests (within 2 months versus more than 2 months): a prolonged interval between two OGTTs may also lead to poor reproducibility even though the optimal interval is still unknown. 5) Age: aging is associated with degradation of the pancreatic β-cells which may affect the accuracy of OGTT.
Meta-regression was conducted to further explore the heterogeneity quantitatively among the studies and to determine the diagnostic accuracy of the OGTT in different conditions. The lnDOR was used as the dependent variable. The standard error of the lnDOR was used to measure the within-study variability, and the residual maximum likelihood method to estimate the between-study variance. Factors associated with significantly different SEN and/or SPE in the subgroup analysis were included in the meta-regression model as covariates.
The potential publication bias was assessed using the Deeks funnel plots[16]. The studies by Eschwege et al. and De Vegt F et al. had much larger sample sizes and much longer intervals between the administrations of the two OGTTs, compared with the other studies included in the meta-analysis (Table1). In order to assess the effect of this study on the pooled result, a post-hoc sensitive analysis was performed to calculate DOR for ACS in the meta-regression model without the studies by Eschwege et al. and De Vegt F et al. In addition, the diagnostic criteria for DM have changed over time, thus the criteria used in the included studies has varied. Another post-hoc sensitive analysis was performed in order to calculate DOR for ACS in the meta-regression model without the study using the 1985 WHO criteria.
All analyses were performed using STATA version 11.0 (Stata Corp; College Station, TX). All statistical tests were two-sided, with a p value of 0.05 denoting statistical significance.
Results
Characteristics and methodological quality of included studies
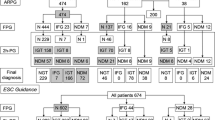
Of the 5,521 references identified in the initial search, only 18 reports fulfilled the criteria for inclusion in the meta-analysis. After communicating with the authors, 15 reports offered sufficient data to build a two-by-two table and thus were included in the final meta-analysis (Figure1)[17–31]. Of the 15 included studies involving 8,027 subjects, 10 studies included patients with ACS, and the remaining consisted of non-ACS individuals. The characteristics of the included studies are detailed in Table1. The results on the methodological quality of the included studies are presented in text form in Additional file1 and in a graph in Figure2.
Pooled results and hierarchic summary ROC curve
The SEN of the included studies ranged from 0.38 to 0.96, whereas the SPE ranged from 0.64 to 0.98 (forest plots in Figure3). The hierarchical summary ROC curve represents the relationship between sensitivity and specificity across the included studies with a 95% confidence ellipse and a 95% prediction ellipse (Figure4). The area under the summary ROC curve (AUC) was 0.87 (95% CI, 0.16-1.00). Using the bivariate model, the pooled results for SEN, SPE, PLR, NLR, and DOR were 0.70 (95% CI, 0.60-0.78), 0.91 (95% CI, 0.86-0.94), 7.6 (95% CI, 4.9-11.7), 0.33 (95% CI, 0.25-0.45), and 23 (95% CI, 12–41), respectively. The I2 value of all measures was 99% (95% CI, 98-99%), indicating significant heterogeneity across the included studies.
Subgroup analysis, meta-regression and publication bias
The subgroup analyses demonstrated that the OGTT performed in ACS patients has similar SEN (0.71 [95%CI, 0.60-0.82] versus 0.67 [95% CI, 0.54-0.81], p=0.43) but a slightly lower SPE (0.86 [95% CI, 0.81-0.92] versus 0.95 [95% CI, 0.93-0.98], p<0.01) compared with non-ACS patients. A prolonged interval between repeated tests (more than 2 months) is also associated with lower SEN (0.62 [95% CI, 0.50-0.73] versus 0.77 [95% CI, 0.68-0.86], p<0.01) and SPE (0.90 [95% CI, 0.84-0.95] versus 0.92 [95% CI, 0.87-0.97], p<0.01). Compared with the younger age (< 60 years) group, advanced age (≥60 years) is associated with lower SPE (0.89 [95% CI, 0.82-0.96] versus 0.92 [95% CI, 0.87-0.97], p=0.01) while the SEN is similar (0.73 [95% CI, 0.62-0.84] versus 0.63 [96% CI, 0.48-0.77], p=0.81). However, using a different threshold (2-hour OGTT with or without FBG) or blood sample (plasma glucose or capillary glucose) did not lead to different diagnostic accuracy (all p>0.05) (Figure5).
Since ACS, interval between repeated tests and age were found to be associated with different SEN and/or SPE in the subgroup analysis, multiple meta-regressions were performed to further determine the effect of these factors on the DOR. However, none of these covariants was found to be associated with different diagnostic accuracy in the multiple meta-regression model (Table2).
The Deeks funnel plot asymmetry test showed insignificant publication bias (p=0.24, Figure6).
The post hoc sensitive analysis, which excluded the largest studies by Eschwege et al. and De Vegt et al., demonstrated a similar result (DOR 0.91, 95% CI 0.02-47.95, p=0.955), indicating the final conclusion of our meta-analysis was not markedly affected by these studies. Another post hoc sensitive analysis excluded studies using the 1985 WHO criteria also demonstrated a result similar to the final conclusion of the meta-analysis (DOR 0.53, 95% CI 0.01-19.36, p=0.687).
Discussion
The role of the OGTT in the diagnosis of diabetes in the general population has been the subject of debate for decades due to the test’s poor reproducibility, which is mainly caused by random variants in glucose metabolism. Thus, it is necessary to take the reproducibility of the OGTT into consideration when evaluating the use of the test in ACS. To the best of our knowledge, this is the first meta-analysis designed to evaluate the accuracy of the OGTT in ACS and to compare it with that in non-ACS cases. In patients with ACS, OGTT has a slightly lower SPE in diagnosing diabetes compared with those without ACS, while the SEN values are comparable. A lower SPE (true negative rate) indicates that performing an OGTT in patients with ACS will result in a higher proportion of false positive results. According to the result of the subgroup analysis, less than one in every 10 ACS patients diagnosed with diabetes using an OGTT before discharge will have a different result at the follow-up OGTT resulting in a change in diagnosis. After adjusting the interval between repeated tests and age in the multiple meta-regression model, performing an OGTT in ACS patients was not associated with lower diagnostic accuracy compared with non-ACS patients. This means that it is reasonable to perform an OGTT to screen for diabetes in patients with ACS before discharge.
It is believed that aging is associated with function of the pancreatic β cells, which may affect the accuracy of OGTT. In the subgroup analysis, advanced age (≥60 years) was associated with lower SPE, while the SEN is similar when compared with the younger age (< 60 years) group. The younger patients, with well preserved function of the pancreatic β cells, maintained their plasma glucose in normal range in the setting of stress and only those with previous unknown DM could be identified during ACS. On the other hand, due to compromised function of the pancreatic β cells, the patients of advanced age were unable to maintain the plasma glucose level during the stress and the plasma glucose returned to the normal range only after the stress was eliminated, which made the OGTT less accurate in patients of advanced age enduring ACS. However, the effect of age on the accuracy of OGTT during ACS is not observed when using the meta-regression model.
Due to the poor reproducibility of the OGTT, it may be reasonable to repeat the test after discharge, which is also recommended in the WHO 1999 criteria[13]. Although the optimal time to repeat an OGTT is still unknown, OGTT could be repeated in 3 months after hospital discharge if necessary, given most of the studies repeated the OGTT 3 months after discharge.
Hyperglycemia during ACS was thought to be “stress hyperglycemia”, which develops due to a highly complex interplay between hormones (such as catecholamines, growth hormones, and cortisol) and cytokines, ultimately leading to excessive hepatic glucose production and insulin resistance[32]. A study by Choi et al. showed that acute myocardial infarction (MI) patients with IGT or diabetes exhibited higher levels of high-sensitivity C-reactive protein and interleukin-6 levels compared with acute MI patients with normal glucose tolerance or well-controlled diabetes, indicating that glycol metabolism in acute MI is associated with acute stress and inflammation[26]. However, this finding was not supported by the other studies included in this meta-analysis. Neither C-reactive protein nor the extent of myocardial damage was found to be related to hyperglycemia in ACS patients[21, 23, 24]. Thus, the evidence of stress hyperglycemia in ACS is still equivocal.
It is well-known that in-hospital hyperglycemia is associated with both the short-term and long-term prognosis in AMI patients[33, 34]. However, either hyperglycemia on admission, fasting plasma glucose or HbA1c, in non-diabetic patients with AMI are not sensitive enough to uncover previously undiagnosed abnormal glucose tolerance or diabetes[35–37]. Furthermore, previous studies have indicated that the 2-h post challenge plasma glucose level was a significant predictor of cardiovascular events in patients with previous MI[38] and the prognosis of AMI patients with a new diagnosis of diabetes or impaired glucose tolerance defined by an OGTT during hospitalization was significantly worse than that of patients with impaired fasting glucose and normal glucose regulation[39–41]. Individuals who had normoglycemia, but whose 2-hour plasma glucose concentrations did not return to the FPG levels during an OGTT have been shown to have increased risk of cardiovascular diseases[42]. Thus, early performance of OGTT during the AMI hospitalization may provide an opportunity to detect the high risk population and establish the undiagnosed diabetes or impaired glucose tolerance. A recent study has shown that discontinuation of anti-hyperglycemic therapy during AMI hospitalization is common and associated with higher mortality rates after discharge in older patients[43]. Early diagnosis of diabetes during AMI hospitalization may be vital to improve compliance with life style changes or anti-hyperglycemic therapy.
Studies of repeated OGTT performed only in patients with abnormal or normal OGTT results in the first test were excluded from this meta-analysis, since these studies may lead to underestimation or overestimation of the accuracy of OGTT. Studies with intended medical intervention between the two OGTTs were also excluded, because several interventions such as exercise and medication have been shown to be associated with improved glucose tolerance.
The use of the hemoglobin A1C test to diagnose DM, with a threshold of 6.5%, had been recommended by ADA 2010 criteria due to its stability and convenience[44]. Recently, Ramachandran et al. divided newly diagnosed DM patients with ACS into two groups based on A1C level: undiagnosed preexisting diabetes was considered possible if the hemoglobin A1C values were ≥6.0%, and stress hyperglycemia was considered if the hemoglobin A1C values were <6.0%. Surprisingly, after 3 months, all of the undiagnosed preexisting diabetes patients remained diabetic, while only 16.7% of the stress hyperglycemia patients remained diabetic[45]. Thus, new consensus statements on the care of the hyperglycemia in ACS patients have recommended its use during hospitalization with an OGTT[46].
The studies have several limitations. First, even though the analysis identified statistically insignificant publication bias, all of the articles that fulfilled the inclusion criteria were not included because some authors were unwilling to offer the original data and there may be data relevant to this topic which have never been published. Second, these findings are based on an indirect comparison of two groups of participants, and no comparison of the reproducibility of the OGTT between ACS and non-ACS patients was available in a single study. Finally, this is a meta-analysis of study level, instead of individual level, which makes it impossible for us to determine the optimal timing for OGTT during hospitalization.
Conclusion
Based on the meta-analysis, performing an OGTT in ACS has similar diagnostic accuracy with that in non-ACS cases. It is reasonable to use the OGTT to screen for diabetes during the hospital stay of ACS patients. Further studies should focus on the optimal timing of OGTT during ACS hospitalization.
Abbreviations
- OGTT:
-
Oral glucose tolerance test
- DM:
-
Diabetes mellitus
- ACS:
-
Acute coronary syndrome
- SPE:
-
Sensitivity
- SPE:
-
Specificity
- PLR:
-
Positive likelihood ratio
- NLR:
-
Negative likelihood ratio
- DOR:
-
Diagnostic odds ratio
- CI:
-
Confidence interval
- NICE:
-
National Institute for Health and Clinical Excellence
- WHO:
-
World Health Organization
- ADA:
-
American Diabetes Association
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the summary ROC curve
- MI:
-
Myocardial infarction.
References
Bartnik M, Rydén L, Ferrari R, Malmberg K, Pyörälä K, Simoons M, Standl E, Soler-Soler J, Ohrvik J, Euro Heart Survey Investigators: The prevalence of abnormal glucose regulation in patients with coronary artery disease across europe. The euro heart survey on diabetes and the heart. Eur Heart J. 2004, 25: 1880-1890. 10.1016/j.ehj.2004.07.027.
Hu DY, Pan CY, Yu JM: The relationship between coronary artery disease and abnormal glucose regulation in china: The china heart survey. Eur Hear J. 2006, 27: 2573-2579. 10.1093/eurheartj/ehl207.
Capes SE, Hunt D, Malmberg K, Gerstein HC: Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet. 2000, 355: 773-778. 10.1016/S0140-6736(99)08415-9.
Angeli F, Verdecchia P, Karthikeyan G, Mazzotta G, Del Pinto M, Repaci S, Gatteschi C, Gentile G, Cavallini C, Reboldi G: New-onset hyperglycemia and acute coronary syndrome: A systematic overview and meta-analysis. Curr Diabetes Rev. 2010, 6: 102-110. 10.2174/157339910790909413.
Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A: Guidelines on diabetes, pre-diabetes, and cardiovascular diseases, et al: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007, 28: 88-136.
Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, ESC Committee for Practice Guidelines (CPG): Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008, 29: 2909-2945.
Senthinathan A, Kelly V, Dzingina M, Jones D, Baker M, Longson D: Hyperglycaemia in acute coronary syndromes: summary of NICE guidance. BMJ. 2011, 343: d6646-10.1136/bmj.d6646.
Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P: Hyperglycemia and acute coronary syndrome: a scientific statement from the American heart association diabetes committee of the council on nutrition, physical activity, and metabolism. Circulation. 2008, 117: 1610-1619. 10.1161/CIRCULATIONAHA.107.188629.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB: Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000, 283: 2008-2012. 10.1001/jama.283.15.2008.
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J: The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003, 3: 25-10.1186/1471-2288-3-25.
Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985, 727: 1-113.http://www.ncbi.nlm.nih.gov/pubmed?term=World%20%20Health%20%20Organ%20%20Tech%20%20Rep%20Ser%201985%2C%20727%3A1%E2%80%93113.
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003, 26 (Suppl 1): S5-20.
World Health Organization, IDF: Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Report of a WHO/IDF consultation. 2006, 1-50.http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/index.html.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH: Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005, 58: 982-990. 10.1016/j.jclinepi.2005.02.022.
Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ. 2003, 327: 557-560. 10.1136/bmj.327.7414.557.
Deeks JJ, Macaskill P, Irwig L: The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005, 58: 882-893. 10.1016/j.jclinepi.2005.01.016.
Wei F: The clinical analysis of glycometabolic disturbance in patients with acute coronary syndrome. Int J Cardiol. 2011, 152: S112-abstract
Ilany J, Michael L, Cohen O, Matetzky S, Gorfine M, Hod H, Karasik A: Glucose homeostasis abnormalities assessed by an OGTT in coronary artery disease patients during admission and follow-up at ambulation. Exp Clin Endocrinol Diabetes. 2011, 119: 463-466. 10.1055/s-0031-1271668.
Bronisz A, Kozinski M, Magielski P, Fabiszak T, Gierach J, Swiatkiewicz I, Sukiennik A, Kubica A, Bronisz M, Grabczewska Z, Sinkiewicz A, Junik R, Kubica J: Value of oral glucose tolerance test in the acute phase of myocardial infarction. Cardiovasc Diabetol. 2011, 10: 21-10.1186/1475-2840-10-21.
Jiménez-Navarro MF, Garcia-Pinilla JM, Garrido-Sanchez L, Alonso-Briales JH, Pérez-Cabeza A, Ortiz-García C, Hernández-Garcia JM, Tinahones F, de Teresa E: Poor reproducibility of the oral glucose tolerance test in the diagnosis of diabetes during percutaneous coronary intervention. Int J Cardiol. 2010, 142: 245-249. 10.1016/j.ijcard.2009.01.020.
Lewczuk A, Hirnle T, Sobkowicz B, Sawicki R, Tomaszuk-Kazberuk A, Knapp M, Musial W, Juszczyk G, Jakubow P, Cydzik M: Glucose metabolism abnormalities after acute myocardial infarction in patients with one-vessel coronary artery disease, treated with primary percutaneous coronary intervention-1-year follow-up. Przeglad Kardiodiabetologinczny. 2009, 4: 3-10.
Knudsen EC, Seljeflot I, Abdelnoor M, Eritsland J, Mangschau A, Arnesen H, Andersen GO: Abnormal glucose regulation in patients with acute ST-elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol. 2009, 8: 6-10.1186/1475-2840-8-6.
Srinivas-Shankar U, Somauroo JD, Delduca AM, Jordan TS, Bowles SA, Rutter MK: Temporal change in glucose tolerance non-ST-elevation myocardial infarction. Diabetes Res Clin Pract. 2008, 82: 310-316. 10.1016/j.diabres.2008.08.016.
Lankisch M, Füth R, Gülker H, Lapp H, Bufe A, Haastert B, Martin S, Rathmann W: Screening for undiagnosed diabetes in patients with acute myocardial infarction. Clin Res Cardiol. 2008, 97: 753-759. 10.1007/s00392-008-0674-5.
Liu M, Pan CY, Jin MM, Su HY, Lu JM: The reproducibility and clinical significance of oral glucose tolerance test for abnormal glucose metabolism. Zhonghua Nei Ke Za Zhi. 2007, 46: 1007-1010.
Choi KM, Lee KW, Kim SG, Kim NH, Park CG, Seo HS, Oh DJ, Choi DS, Baik SH: Inflammation, insulin resistance, and glucose intolerance in acute myocardial infarction patients without a previous diagnosis of diabetes mellitus. J Clin Endocrinol Metab. 2005, 90: 175-180.
Tenerz A, Norhammar A, Silveira A, Hamsten A, Nilsson G, Ryden L, Malmberg K: Diabetes, insulin resistance, and the metabolic syndrome in patients with acute myocardial infarction without previously known diabetes. Diabetes Care. 2003, 26: 2770-2776. 10.2337/diacare.26.10.2770.
Eschwege E, Charles MA, Simon D, Thibult N, Balkau B: Reproducibility of the diagnosis of diabetes over a 30-month follow-up: The paris prospective study. Diabetes Care. 2001, 24: 1941-1944. 10.2337/diacare.24.11.1941.
De Vegt F, Dekker JM, Stehouwer CDA, Nijpels G, Bouter LM, Heine RJ: Similar 9-year mortality risks and reproducibility for the World Health Organization and American Diabetes Association glucose tolerance categories: the Hoorn Study. Diabetes Care. 2000, 23: 40-44. 10.2337/diacare.23.1.40.
Ko GTC, Chan JCN, Woo J, Lau E, Yeung VTF, Chow CC, Cockram CS: The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998, 35: 62-67.
Farrer M, Fulcher G, Albers CJ, Neil HAW, Adams PC, Alberti KGMM: Patients undergoing coronary artery bypass graft surgery are at high risk of impaired glucose tolerance and diabetes mellitus during the first postoperative year. Metabolism. 1995, 44: 1016-1027. 10.1016/0026-0495(95)90099-3.
Dungan KM, Braithwaite SS, Preiser JC: Stress hyperglycaemia. Lancet. 2009, 373: 1798-1807. 10.1016/S0140-6736(09)60553-5.
Goyal A, Mehta SR, Díaz R, Gerstein HC, Afzal R, Xavier D, Liu L, Pais P, Yusuf S: Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation. 2009, 120: 2429-2437. 10.1161/CIRCULATIONAHA.108.837765.
Pinto DS, Kirtane AJ, Pride YB, Murphy SA, Sabatine MS, Cannon CP, Gibson CM, CLARITY-TIMI 28 Investigators: Association of blood glucose with angiographic and clinical outcomes among patients with ST-segment elevation myocardial infarction (from the CLARITY-TIMI-28 study). Am J Cardiol. 2008, 101: 303-307. 10.1016/j.amjcard.2007.08.034.
Tenerz A, Lönnberg I, Berne C, Nilsson G, Leppert J: Myocardial infarction and prevalence of diabetes mellitus. Is increased casual blood glucose at admission a reliable criterion for the diagnosis of diabetes?. Eur Heart J. 2001, 22: 1102-1110. 10.1053/euhj.2000.2445.
Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Hata T, Nakama Y, Kijima Y, Kagawa E: Is admission hyperglycaemia in non-diabetic patients with acute myocardial infarction a surrogate for previously undiagnosed abnormal glucose tolerance?. Eur Heart J. 2006, 27: 2413-2419. 10.1093/eurheartj/ehl271.
de Mulder M, Oemrawsingh RM, Stam F, Boersma E, Umans VA: Comparison of diagnostic criteria to detect undiagnosed diabetes in hyperglycaemic patients with acute coronary syndrome. Heart. 2012, 98: 37-41. 10.1136/heartjnl-2011-300163.
Henareh L, Agewall S: 2-h postchallenge plasma glucose predicts cardiovascular events in patients with myocardial infarction without known diabetes mellitus. Cardiovasc Diabetol. 2012, 11: 93-10.1186/1475-2840-11-93.
Mazurek M, Kowalczyk J, Lenarczyk R, Sredniawa B, Zielinska T, Sedkowska A, Jedrzejczyk E, Pruszkowska P, Polonski L, Kalarus Z: Independent mortality predictors in patients with different glucose abnormalities and acute myocardial infarction treated invasively. Eur Heart J. 2011, 32: 974.
Kitada S, Otsuka Y, Kokubu N, Kasahara Y, Kataoka Y, Noguchi T, Goto Y, Kimura G, Nonogi H: Post-load hyperglycemia as an important predictor of long-term adverse cardiac events after acute myocardial infarction: a scientific study. Cardiovasc Diabetol. 2010, 9: 75-10.1186/1475-2840-9-75.
Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L: Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004, 25: 1990-1997. 10.1016/j.ehj.2004.09.021.
Ning F, Zhang L, Dekker JM, Onat A, Stehouwer CD, Yudkin JS, Laatikainen T, Tuomilehto J, Pyörälä K, Qiao Q, DECODE Finnish and Swedish Study Investigators: Development of coronary heart disease and ischemic stroke in relation to fasting and 2-hour plasma glucose levels in the normal range. Cardiovasc Diabetol. 2012, 11: 76-10.1186/1475-2840-11-76.
Lipska KJ, Wang Y, Kosiborod M, Masoudi FA, Havranek EP, Krumholz HM, Inzucchi SE: Discontinuation of antihyperglycemic therapy and clinical outcomes after acute myocardial infarction in older patients with diabetes. Circ Cardiovasc Qual Outcomes. 2010, 3: 236-242. 10.1161/CIRCOUTCOMES.109.887620.
American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010, 33 (Suppl 1): S62-69.
Ramachandran A, Chamukuttan S, Immaneni S, Shanmugam RM, Vishnu N, Viswanathan V, Jaakko T: High incidence of glucose intolerance in asian-indian subjects with acute coronary syndrome. Diabetes Care. 2005, 28: 2492-2496. 10.2337/diacare.28.10.2492.
Vergès B, Avignon A, Bonnet F, Catargi B, Cattan S, Cosson E, Ducrocq G, Elbaz M, Fredenrich A, Gourdy P, Henry P, Lairez O, Leguerrier AM, Monpère C, Moulin P, Vergès-Patois B, Roussel R, Steg G, Valensi P, Diabetes and Cardiovascular Disease study group of the Société francophone du diabète (SFD): Société française de cardiologie (SFC): Consensus statement on the care of the hyperglycaemic/diabetic patient during and in the immediate follow-up of acute coronary syndrome. Arch Cardiovasc Dis. 2012, 105: 239-253.
Acknowledgement
There is no financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YY participated in study design, literature search, data analysis, interpretation, and writing. HX participated in study design, literature search, data analysis, interpretation, and writing. XZ participated in study design, literature search, data analysis, interpretation, and writing. SZ conceived of the study, data analysis, data interpretation, and writing. All authors read and approved the final manuscript.
Yicong Ye, Hongzhi Xie, Xiliang Zhao contributed equally to this work.
Electronic supplementary material
12933_2012_564_MOESM1_ESM.pdf
Additional file 1: It includes the details of the search strategy and the assessment of study quality (risk of bias). (PDF 31 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ye, Y., Xie, H., Zhao, X. et al. The oral glucose tolerance test for the diagnosis of diabetes mellitus in patients during acute coronary syndrome hospitalization: a meta-analysis of diagnostic test accuracy. Cardiovasc Diabetol 11, 155 (2012). https://doi.org/10.1186/1475-2840-11-155
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-11-155