Abstract
Background
The contribution of the processes involved and waste generated during gold mining to the increment of heavy metals concentration in the environment has been well established. While certain heavy metals are required for the normal functioning of an organism, certain heavy metals have been identified for their deleterious effects on the ecosystem and non-physiological roles in organisms. Hence, efforts aimed at reducing their concentration level are crucial. To this end, soil and water samples were collected from Ilesha gold mining, Osun State, Nigeria, and they were subjected to various analyses aimed at evaluating their various physicochemical parameters, heavy metal concentration, heavy metal-resistant bacteria isolation, and other analyses which culminated in the molecular characterization of heavy metal-resistant bacteria.
Results
Notably, the results obtained from this study revealed that the concentration of heavy metal in the water samples around the mining site was in the order Co > Zn > Cd > Pb > Hg while that of the soil samples was in the order Co > Cd > Pb > Hg > Zn. A minimum inhibitory concentration test performed on the bacteria isolates from the samples revealed some of the isolates could resist as high as 800 ppm of Co, Cd, and Zn, 400 ppm, and 100 ppm of Pb and Hg respectively. Molecular characterization of the isolates revealed them as Priestia aryabhattai and Enterobacter cloacae.
Conclusion
Further analysis revealed the presence of heavy metal-resistant genes (HMRGs) including merA, cnrA, and pocC in the isolated Enterobacter cloacae. Ultimately, the bacteria identified in this study are good candidates for bioremediation and merit further investigation in efforts to bioremediate heavy metals in gold mining sites.
Similar content being viewed by others
Background
Various anthropogenic activities, especially mining, have been well reported to negatively affect the environment, these effects are diverse and include the destruction of ecosystems by polluting the aquatic and terrestrial environment and altering soil properties [1]. Apart from the destruction of the physical habitat, mining also causes the loss of biodiversity [2]. Therefore, mining sites cause various toxicological challenges for the surrounding ecosystems and human health. Of all the numerous mining activities ongoing in the world, gold mining activities occur at a high rate due to its economic value. Three basic steps (mining, mineral processing, and metallurgical extraction) are involved in the gold mining process, and over 99% of the ore extracted during gold mining is released into the surrounding environment as waste [3]. Although heavy metals (HM) are elements that occur naturally in the environment, they are however part of the wastes that have been implicated around gold mining sites [4].
Generally, HMs are referred to as elements with atomic weight and a density greater than that of water [5]. They are classified into essential and non-essential based on their functions and importance to biological systems. Notably, essential HM including Copper (Cu), Zinc (Zn), Nickel (Ni), Manganese (Mn), and Iron (Fe) are utilized in various physiological and biochemical functions that are pertinent to the existence and normal functioning of living organisms. Exemplifying this is their role in facilitating enzymatic reactions by serving as enzyme cofactors and their role in the regulation of osmotic balance [6]. Conversely, HMs such as Lead (Pb), Arsenic (As), Cadmium (Cd), Chromium (Cr), and Mercury (Hg) do not perform any physiological role in living organisms; hence, they are referred to as non-essential HMs [7]. Noteworthy, both essential and non-essential HMs are known to pose deleterious effects on plants, animals, microorganisms, and the environment, an effect that has been widely reported to be heavily dependent on the dose and duration [8]. The accumulation of HMs in plants gives rise to numerous adverse effects such as stunted plant growth, reduction in the ability to photosynthesize and undergo mitosis, decrease in enzymatic activity and nutrient intake, as well as chlorosis [9, 10]. The consumption of these plants by humans, in turn, causes negative effects such as immunosuppressive functions, blindness, and neurological damage, as well as playing roles in the etiology of pathologies such as cancer and hypertension [11, 12]. Similarly, the presence of HM in aquatic environments also causes oxidative damage to animals in such environments [13]. HMs also affect the soil and its biota by leading to the loss of soil microbial diversity, change in soil PH and porosity, and reduced microbial enzymatic activities [14].
While exposure to HM results in the loss of soil microbial diversity and reduced enzymatic activities, certain microorganisms particularly bacteria, have developed resistance mechanisms against them, to tolerate the toxic effects they pose. These resistance mechanisms include enzymatic detoxification, exclusion by permeability barrier, intracellular and extracellular sequestration, efflux pumps, active transport, as well as reduction of heavy metal ions and cellular targets [15]. Of note, these resistance mechanisms are often conferred by natural selection or exposure to substrates containing heavy metal ions [16]. Salmonella, Escherichia coli, and Rhodobacter sphaeroides are among the bacteria that have been reported to possess multiple heavy metal resistance genes (HMRGs); hence, they are regarded as suitable for environmental remediation [6, 17].
Profiling of the microorganisms in mining sites could help identify the microorganisms that could be utilized in reducing the concentration of HM in such environments, hence, contributing to the global efforts to reduce pollution caused by HM. Consequently, this study aims to identify microorganisms that are present in the Ilesha gold mining site in Nigeria.
Methods
Study area
This study was carried out on a gold mining site located in Ilesa, Osun State, Nigeria. Ilesa is one of the major cities in Osun State having a geographical coordinate of 7.6103° N (Latitude) and 4.7096° E (Longitude). It has six local governments (Ilesa East, Ilesa West, Obokun, Oriade, Atakunmosa East, and Atakunmosa West) with a total estimated population of 620,109, according to the 2006 population census [18]. Ilesha is commonly known to have large commercializable deposits of gold and numerous illegal gold mining sites. It is also known to produce crops such as cocoa, kola nuts, pumpkins, oil palm, and cotton.
Sample collection
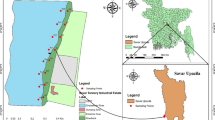
Soil and water samples were collected separately from seven different gold mines located in Ilesa (Fig. 1). The samples were labeled 1A, 1B, 2A, 2B, 3A, 3B, 4A, 4B, 5A, 5B, 6A, 6B 7A, and 7B based on the sampling locations. Table 1 presents the sampling locations and coordinates.
Physicochemical analyses of water and soil samples
Various physicochemical properties of the water samples including pH, chloride, conductivity, total hardness, biochemical oxygen demand (BOD), turbidity, phosphate, sulfate, nitrate, bicarbonate, calcium, potassium, magnesium, and sodium were analyzed [19, 20]. Similarly, the pH, conductivity, organic matter, carbon, nitrogen, phosphorus, calcium, magnesium, potassium, and sodium content of the soil samples were also determined using standard methods [21,22,23].
Heavy metal analysis
Soil digestion
For Zn, Pb, Cd, and Co: the soil samples were pulverized and oven-dried at 500 °C. One gram of the resulting sample was weighed into a 100-mL conical flask and distilled water was added. Subsequently, 10 ml of aqua regia HNO3: HCl (3:1) was added to the mixture and boiled with steady heat to almost dryness. The resulting sample was then allowed to cool and filtered, the filtrate was made up to 100 ml with distilled water and subjected to HM analysis.
For Hg: 0.5 g of each soil sample was digested with 10 mL of concentrated HNO3 until a clear solution was obtained. The digest was filtered in a 50-mL tube and made up to the 50 mL mark with distilled water, after which it was subjected to HM analysis.
Water digestion
For Zn, Pb, Cd, and Co: 100 mL of water sample was measured into a conical flask, and 2 mL of concentrated HNO3 and 5 mL of concentrated HCl were added. The resulting mixture was subjected to heating at 900–950 °C till the volume was reduced to 15–20 mL. Ultimately, the volume of the heated mixture was made up to 100 mL and was subjected to heavy metal analysis.
For Hg: 50 mL of the water samples were digested with 5 mL of HNO3 until the volume was reduced to 20 mL and the volume was made up to 50 mL with distilled water. It was then subjected to heavy metal analysis.
Determination of heavy metals in samples using atomic absorption spectrophotometer (AAS)
An atomic absorption spectrometer (AAS) was utilized to determine the heavy metal content of the samples in accordance with APHA 20th Edition 3111B and 3111D, ASTM D3561, and ASTM D5198. Direct aspiration of the digested liquid sample in an acidic medium into an air/acetylene or nitrous oxide/acetylene flame at specified wavelengths for each of the heavy metals under investigation was carried out to determine the concentrations of heavy metals in the samples [24].
Enumeration of bacterial loads in soil and water samples
One gram of soil and water sample was measured into 100 mL of distilled water each and 1 ml was transferred into another test tube containing 9 mL of sterile distilled after thorough shaking. Ultimately, making a serial dilution of up to 10−4 [25]. One milliliter of the sample from dilution 10−3 was utilized for the pour plate technique to enumerate the microbial load of the samples while the streaking method was used to isolate bacteria colonies of pure culture [26].
The bacterial colony count was determined by multiplying the number of counts with the dilution used and expressed as colony-forming units per milliliter (cfu/mL) of water and colony-forming units per gram (cfu/g) of soil [27].
Characterization of bacterial isolates
The morphological characteristics of the bacteria colonies such as edges, shape, and surface were observed and recorded [28]. The identification and characterization of bacteria isolates were performed by carrying out grams staining and other relevant biochemical tests which include catalase test, coagulase test, indole production test, citrate utilization test, and sugar fermentation [29, 30].
Minimum inhibitory concentration (MIC) test
The minimum inhibitory concentration (MIC) tests of heavy metals were performed using the broth macrodilution method of the Clinical and Laboratory Standards Institute [31]. The individual isolate was cultured in nutrient broth for 18–20 h at 37 °C. The bacterial suspension was diluted to a 0.5 McFarland standard with sterile saline water and inoculated into a media containing different concentrations of the heavy metal salts. Different Part per million (PPM) concentrations of the following heavy metal salts were prepared for the test: mercury chloride (HgCl2), cobalt chloride (CoCl2), cadmium chloride (CdCl2), zinc sulfate (ZnSo4), and lead acetate [Pb(C2H3O2)2]. Concentrations of 12.5, 25, 50, 100, 200, 400, 600, and 800 ppm were used for cadmium, cobalt, zinc and lead respectively [16], while concentrations of 6.25, 12.5, 25, 50, 100 and 200 ppm were used for mercury. After inoculating each isolate to media containing different concentrations of heavy metal salt, the mixtures were incubated at 35 °C for 18–20 h and MIC was recorded as the lowest concentration that visibly inhibits bacterial growth [16]. Positive and negative controls were also prepared for this test. The positive control consisted of the medium and the bacteria isolate, while the negative control consisted of the medium and the heavy metal salt only [32].
DNA extraction and PCR amplification of HMRGs
DNA was extracted from the bacteria isolates of the bacteria that were able to grow in high concentrations of heavy metals using the ZymoBIOMICS™ DNA Miniprep kit [16]. The genotyping for the heavy metal resistance gene in isolated bacteria was performed by PCR using gene-specific forward and reverse primers having similar annealing temperatures of 57 °C (Table 2). The methods and PCR primers (Table 3) used were selected from a previously published study [16]. The PCR products were subjected to gel electrophoresis using 1.5% agarose gels and were viewed on a gel documentation system.
Molecular identification of bacteria isolates and phylogenetic analysis
Genomic DNA was extracted from the cultures using the Quick-DNA™ Fungal/Bacterial Miniprep kit (Zymo research catalog number D6005). The 16S rRNA target region was amplified using OneTaq Quick-load 2X Master Mix 9NEB, (Catalogue number M0486) with primers presented in Table 3. The PCR products were run on a gel and cleaned up enzymatically using the EXOSAP method. The purified fragments were sequenced in the forward and reverse direction using the ABI 3500XL Genetic Analyzer (Applied Biosystems, ThermoFisher Scientific). BioEdit Sequence Alignment Editor Version 7.2.5 was used to analyze the ab1 files generated by the ABI 3500XL Genetic Analyzer and the results obtained were analyzed by BLASTn search. Subsequently, Molecular Evolutionary Genetics Analysis (MEGA) 11 software was employed to perform phylogenetic analysis using the Test Maximum Likelihood method and the bootstrap consensus tree was inferred from 100 replicates [33].
Statistical analysis
The statistical analysis of results obtained was done using Statistical Package for Social Sciences (SPSS), version 25 using analysis of variance (ANOVA) on Windows 10 at a confidence level of 95%.
Results
Physicochemical properties of water samples
The results of the analysis of various physicochemical parameters carried out on the water samples are presented in Table 4. Notably, the pH values of the water samples ranged from 6.2 to 7.2 with sample 1 having the highest pH value of 7.2 while sample 2 had the lowest pH value of 6.2 (Table 4). Analysis of the conductivity of the water samples revealed the conductivity values ranged from 84.4 µS/cm to 540 µS/cm, with Samples 6B and 5A having the lowest and highest values respectively (Table 4). The result of the analysis of the biochemical oxygen demand (BOD) of the water samples revealed the values ranged from 50.0 Mg/L to 108.5 Mg/L, with samples 5A and 5B having the lowest and the highest values respectively. The turbidity values of samples 1, 3B, 4A, 4B, 5B, 6B, and 7A were the lowest with a value of 10.0 Nephelometric turbidity unit (NTU), while sample 5A was the highest with a value of 28.5 NTU. The results of the total hardness of the water samples ranged from 0.32 to 1.00 mg/L, the lowest and the highest values recorded were from samples 6A and samples 4A respectively (Table 4).
Anion (Mg/L)
Further analysis of the water samples for the presence of phosphate (PO43−) revealed samples 3A, 3B, 4A, 4B, 5B, 6B, and 7A had the lowest phosphate concentration with all having the same value of 0.2 Mg/L, while sample 5A had highest concentration with a value of 2.31 Mg/L. The concentration of sulfate (SO42−) in the water samples ranged from 110 Mg/L to 240 Mg/L, samples 2 and 7B had the lowest concentration while sample 5A had the highest concentration (Table 4). The lowest nitrate (NO3−) concentration was observed in sample 5A while sample 2 had the highest values of 22 Mg/L and 89 Mg/L respectively. The highest value observed for bicarbonate (HCO3−) in water samples was that of sample 5A with 590 Mg/L while the lowest concentration was for sample 2 with a value of 300 Mg/L (Table 4). The concentration of chloride ion (Cl−) in water samples ranged from 1.6 to 3.4 Mg/L with sample 5B having the lowest concentration of chloride ion while samples 2 and 5A had the highest chloride concentration.
Cations (Mg/L)
The concentration of sodium ion (Na+) in water samples ranged from 10.5 to 50.0 Mg/L, with sample 2 having the highest Na+ concentration of 50.0 Mg/L while sample 5A had the lowest Na+ concentration of 10.5 Mg/L. The highest potassium ion (K+) concentration was observed in sample 5A with a value of 22 Mg/L while the lowest concentration was observed in sample 1 with a value of 4 Mg/L. Further analysis also revealed sample 5A had the highest concentration of calcium ions with a value of 212 Mg/L while sample 3B had the lowest concentration with a value of 51 Mg/L. The highest magnesium (Mg2+) concentration of 118 Mg/L was observed in sample 5A and the lowest Mg2+ of 46 Mg/L was observed in sample 3B (Table 4).
Physicochemical properties of soil samples
Table 5 presents the results of the various physicochemical analyses carried out on the soil samples. The pH values of the soil samples ranged from 5.1 to 6.9 with sample 7B having the highest pH value of 6.9, while sample 3A had the lowest pH value of 5.1. The conductivity of the soil samples ranged from 17.8 to 126.1 µS/cm, with samples 6A and samples 7A having the lowest and the highest conductivity values respectively. Sample 6B was observed to possess the highest percentage carbon concentration while sample 5B had the lowest value. The percentage concentration of organic matter ranged from 0.30 to 0.83%, with samples 4A and 7A having the lowest and the highest values respectively (Table 5). The percentage concentration of nitrogen in the soil samples ranged from 0.02 to 0.26%. Notably, samples 4A, 4B, 5A, 5B, and 6A had the lowest values while sample 3A had the highest value. Sample 6B had the highest phosphorus concentration of 6.28 Mg/Kg, while sample 3B had the lowest concentration of phosphorus with 4.0 Mg/Kg.
Cations (CmolKg−1)
The concentration of Ca2+ in the samples ranged from 0.79 to 1.12 CmolKg−1, with sample 4A having the lowest concentration while samples 1B and 3B had the highest concentration. The concentration of Mg2+ in the soil samples ranged from 0.55 to 0.73 CmolKg−1 with samples 7A and Sample 1B having the lowest and the highest values respectively. The highest concentration of K+ in soil samples was 0.32 CmolKg−1 and was observed in sample 6A, while sample 4B had the lowest concentration of K+ with 0.20 CmolKg−1. The highest concentration of Na+ was observed in sample 1B with a value of 0.23 CmolKg−1 while the lowest concentration was observed in samples 6B and 7A with a value of 0.12 CmolKg−1.
Heavy metal analysis of water samples
The results of the analysis of the heavy metals concentration in the water samples are presented in Table 6. The result revealed that the concentration of Cd in the water samples ranged from 0.006 ppm to 0.072 ppm, with samples 4A and 4B having the lowest and highest values respectively. The highest concentration of cobalt (Co) in water samples was observed in samples 2 and 5B with values of 0.036 ppm, while the lowest concentration was observed in sample 7B with a value of 0.004 ppm. Similarly, sample 7B had the lowest concentration of Pb while sample 2 had the highest concentration with 0.183 ppm. The concentration of zinc in the water samples ranged from 0.119 ppm to 0.275 ppm with sample 4A and sample 2 having the lowest and the highest concentration. The concentration of mercury in water samples ranged from 0.516 ppm to 0.658 ppm with sample 5A and sample 7A having the lowest and the highest concentration respectively.
Heavy metal analysis of soil samples
Table 7 presents the result of the heavy metal concentrations in the soil samples. Sample 2B was observed to have the highest Cd concentration of 0.146 ppm and sample 7A had the lowest Cd concentration of 0.016 ppm. The concentration of Co ranged from 0.012 ppm which was observed in samples 3A and 6A, to 0.078 ppm which was observed in sample 5A. Sample 2B was observed to possess the highest Pb concentration of 0.612 ppm while sample 7A was observed to possess the lowest Pb concentration of 0.109 ppm. The concentration of Zn in the soil samples ranged between 0.298 ppm to 1.221 ppm with samples 7B and 2A having the lowest and the highest concentration respectively. The concentration of mercury in soil samples ranged from 0.309 ppm observed in sample 7B to 0.796 ppm observed in sample 5A.
Enumeration of bacterial loads in soil and samples
The results of the mean bacterial load of each soil and water sample on nutrient agar are presented in Fig. 2. For the soil samples, the results show that sample 5B had the highest bacteria load of 11.67 ± 0.67 × 104 CFU/g, and soil sample 2B had the lowest bacteria load of 2.57 ± 0.33 × 104 CFU/g. Conversely, water sample 7A had the highest bacteria load of 4.70 ± 0.58 × 104 CFU/mL, and the lowest bacteria load of 1.70 ± 0.58 × 104 CFU/mL was observed in sample 4A (Fig. 2).
Characterization of bacterial isolates
A total of ten bacteria isolates were purified and isolated from the soil and water samples. Table 8 presents the result of the analysis of the morphological characteristics of the bacteria isolated from the soil and water samples. Notably, isolates 1 and 3 are gram-negative cocci bacteria with creamy white colonies and also negative for spore formation. Isolates 2, 6, 8, and 10 are gram-positive rod bacteria that were also positive for spore formation. Isolate 4 is a gram-positive cocci bacterium, positive for spore formation and creamy white. Isolates 5, 7, and 9 are gram-negative rod bacteria and are also negative for spore formation (Table 8). The probable identity of the 10 bacteria isolates was determined after subjecting the isolates to Gram’s stain reaction and various biochemical tests including coagulase, catalase, indoles, motility, and sugar fermentation (Table 9). The occurrence and distribution of isolates are presented in Supplementary Tables S1 and S2.
Minimum inhibitory concentration (MIC)
All 10 bacteria isolates were subjected to a minimum inhibitory concentration test to determine the lowest concentration of heavy metals that visibly inhibits the growth of each bacterial isolate. The MIC values of lead, cobalt, cadmium, zinc, and mercury for bacteria isolates are presented in Table 10.
PCR amplification of heavy metals resistance genes (HMRGs)
DNA samples were extracted from four bacteria isolates including isolates 2, 6, 7, and 9. DNA was extracted from these four bacteria isolates because they were able to grow at high concentrations of heavy metals that they were subjected to. Isolate 2, 6, 7, and 9 were all genotyped for HMRGs with a focus on merA (mercury), pcoC (copper), pbrT (lead), cadD (cadmium), and cnrA (cobalt and nickel). The merA, pcoC, pbrT, cadD, and cnrA genes amplified at 1011 bp, 333 bp, 448 bp, 155 bp, and 422 bp respectively. Only isolate 7 amplified for merA, cnrA, and pocC while all other isolates 2, 6, and 9 did not amplify for any of the HMRGs (Fig. 3A, B).
Amplified HMRGs in Isolated Bacteria 2, 6, 7, and 9. Key: A (amplified mercury HMRGs in isolate 7); B (amplified cobalt, nickel, and copper HMRGs in isolate 7); 1: (Isolate 2) 2: (Isolate 6) 3: (Isolate 7) 4: (Isolate 9); Hg (mercury); Pb (lead); Cd (cadmium); Cu (copper); CoN (cobalt and nickel), M: DNA marker/ladder (1 Kb)
Molecular identification by 16S rRNA analysis
A total of three isolates (isolates 6, 7, and 9) were subjected to 16S rRNA sequencing, and the identities of the isolates are presented in Table 11. The 16S rRNA sequencing and BLAST result confirmed the identity of isolate 6 to be Priestia aryabhattai B8W22 while isolates 7 and 9 are Enterobacter cloacae subsp. dissolvens strain LMG 2683 (Table 11).
As presented in Table 11 and Fig. 4, isolate 6 was found to bear the closest phylogenetic relationship to Priestia aryabhattai B8W22 with a percentage identity of 98.53% in the 99% sequence covered while isolates 7 and 9 were found to bear the closest phylogenetic relationship to Enterobacter cloacae subsp. dissolvens strain LMG 2683 with a percentage identity of 89.17% and 96.59% in the 99% and 100% sequence that was covered in their respective query.
Discussion
HM contamination of water and soil due to various precious metal mining activities is a huge source of concern due to its potential effect on public health. In this study, soil and water samples from seven different gold mining sites in Ilesha were studied for their HM contamination and HM-resistant bacteria. Analysis of the physicochemical properties of the soil and water samples revealed the values of the pH of all the studied sites ranged between 6.2 to 7.2 while that of the soil samples ranged between 5.1 to 6.9. The pH of the soil samples from some of the study areas was observed to be acidic as reported by Akinfesi et al. for African soils [34], however, the ongoing mining activities were noticed to have increased the pH of some of the study areas most notably site 7B as the pH was found to be approaching the neutral level (Table 5). Further increase of the pH to the alkaline range could result in the accumulation of heavy metals on the soil surface [35]. The range of the values of the pH of the water samples from the study area was found to be within the WHO recommended range except that of sample 2 which was found to be more acidic than the other samples (Table 4). The electrical conductivity (EC) of soil and water samples measured in µS/cm gives the total amount of dissolved salts and minerals present in the water or soil sample. The EC values of the water samples were observed to vary greatly with Sample 5A having the highest value, however, they were all observed to be well below the WHO-recommended value (Table 4). This could be attributed to the higher composition of anions in the water samples which were observed to be much higher than the WHO-recommended value, as well as the lower composition of cations which was also well below the recommended value [36]. For the soil samples, the EC values were observed to be much higher compared to the values reported by Ibrahim et al. but much lower compared to the values reported by Edema et al. in mining sites located in different geographical regions in Nigeria [27, 37]. Spatial variation, varying intensities, and levels of gold mining activities could be the probable reason for this as this phenomenon also affects soil properties within the close range [27, 38]. The biochemical oxygen demand (BOD) of all the water samples was found to be well above the WHO-recommended limit (Table 4). Notably, high BOD values indicate high levels of contaminants and result in bacteria requiring more oxygen to degrade the contaminants [39]. The high BOD values of the samples in this study are suspected to be a result of the discharge of heavy metal tailings from the mining sites. These heavy metal tailings contain heavy metals with low- or non-degradability [40]. Assessment of the turbidity profile of the water samples revealed that the values of the turbidity were at least twice that of the WHO recommended value (Table 4). Similar values were reported by Rakotondrabe et al. in a mining site in Cameroon, and it was attributed to soil leaching and deforestation around the mining areas [38]. The highest percentage of carbon and nitrogen were 0.65 and 0.26 respectively, with some samples having as low as 0.02% nitrogen. This depicts that the soil has poor carbon and nitrogen content which is due to the ongoing mining activities which involve the removal of the topsoil and replacement with the soil beneath, a phenomenon which reduces the carbon and nitrogen content of the soil [41]. In tandem with the results of this study is a study by Ibrahim et al. at a gold mining site in Zamfara, Nigeria, in which similar levels of carbon and nitrogen were reported [27].
The concentration of heavy metals in water and soil samples from the mining site under study was also assessed and the results are presented in Tables 6 and 7. The order of abundance of the heavy metals in the water samples based on the mean values of their concentration was: Co > Zn > Cd > Pb > Hg. Conversely, the order of abundance of the heavy metals in the soil samples based on the mean values of their concentration was: Co > Cd > Pb > Hg > Zn. Notably, values of the concentration of some of the water samples from the mining site were found to be higher than the WHO recommendation, with tailings from gold extraction and chemicals used during the gold extraction process suspected to be the probable sources of these heavy metals [42, 43]. Results of the assessment of the heavy metal concentration in the soil samples were much lower compared to values reported in similar studies conducted on heavily metals polluted soils in Nigeria [44, 45]. However, variation in mining activity levels is suspected to be the reason for the wide differences.
Bacterial counts recorded from the soil and water from the mining sites revealed Sample 5B to possess the highest bacteria load of 11.67 × 104 CFU/g for the soil samples while Sample 7A had the highest bacteria load of 4.70 × 104 CFU/ml. As evident from the results, the bacteria counts obtained from the soil and water samples were low and this is likely to be a result of gold mining activity and deposition of tailings in water samples, hence, resulting in the stifling of the microbial community in the sites. However, the presence of resistant species in the sites cannot be overlooked. Ten heavy metal-tolerant isolates were recovered of which preliminary identification analysis revealed five to be Gram-positive while the other five were Gram-negative. Interestingly, studies have shown that Gram-negative bacteria are more tolerant to heavy metals than Gram-positive bacteria, a phenomenon which has been suspected to be due to the ability of their cell wall to interact with the metal ions on the surface and the interface of the bacteria [28, 46]. Further subjection of the bacteria isolates to minimum inhibitory concentration tests revealed isolates 2, 6, 7, and 9 as the isolates capable of tolerating high concentrations of Pb, Co, Cd, Zn, and Hg as evident in Table 9. Precisely, all the bacteria isolates except isolate 7 were not able to grow in the presence of lead at a concentration of 600 ppm. Most bacteria isolate except isolates 2, 6, 7, and 9 had low level of resistance to mercury with no bacteria growth observed in mercury at a concentration of 200 ppm. Corroborating the result of this study is the study of Rahman and Singh in which they reported that the MIC values of Hg for a range of Hg-resistant bacteria were from 50 to 100 mg/L [47]. The ability of Hg to inhibit the growth of these bacteria isolates could be due to its high level of toxicity [48, 49]. Contrastingly, most of the bacteria isolated were resistant to high concentration of Cd and Co, in tandem with this is the result of the study of Terzi and Civelek in which they observed that high concentration of Cd was still being tolerated by their bacteria isolates [17]. HMRGs namely merA (mercury), pcoC (copper), pbrT (lead), cadD (cadmium), and cnrA (cobalt and nickel) were genotyped in isolates 2, 6, 7, and 9.
Notably, only isolate 7 amplified for merA, cnrA, and pocC while the other HMRGs were not amplified in any of the other isolates. While the results obtained from the amplification revealed just one isolate as expressing the HMRGs, it is worth noting that the expression of HMRGs is not the only mechanism via which bacteria resist HM. Hence, other mechanisms including the modification of their membrane and metabolic adaptation could be the coping mechanism for the bacteria isolates [50]. Preliminary identification analysis suggested that isolates 2, 6, 7, and 9 are Thiobacillus, Bacillus subtilis, Enterobacter, and Pseudomonas plecoglossicida respectively. However, further characterization revealed isolate 6 to be Priestia aryabhattai B8W22 while isolates 7 and 9 were found to be Enterobacter cloacae subsp. dissolvens strain LMG 2683. Priestia aryabhattai, formerly known as Bacillus aryabhattai [51], has been reported to possess heavy metals remediation capacity. In a study by Singh et al., it was reported that the Priestia aryabhattai biovolatalized As and they also reported the presence of multiple ars genes in the chromosomal DNA of the organism [52]. Interestingly, the arsB gene which encodes a transport membrane protein that functions as an efflux pump and extrudes As out of the cell [53], was reported to be present in the organism, arsC, which functions in an operon to act as cytoplasmic reductase and reduces As5+ to As3+ [54], was reported to be present, arsH gene, which has been reported to confer a high level of resistance to As (V) and As (III) [55], was also present. Furthermore, the arsD gene which encodes a metalloid-responsive transcriptional repressor that is responsible for controlling the expression of ars operon [54], arsR gene which is responsible for regulating ars operon in the presence of As (III) were both present in the organism [56], and arsA, an ATPase activated by As (III) were all reported to be present [53]. Also, the organism was reported to upregulate the expression pattern of certain proteins in response to exposure to As, these proteins were found to function in pathways relating to energy metabolism, proline synthesis, and membrane proteins among many others [57]. Hence, it depicts the ability of the bacteria to adjust to As-induced stress conditions. In another study, Priestia aryabhattai was also reported to possess the ability to degrade an organophosphate herbicide in a process mediated by the goxB gene which encodes a FAD-dependent glyphosate oxidase enzyme [58]. Progressively, this organism is worthy of exploration for bioremediating efforts. Similarly, the heavy metals resistant ability of Enterobacter cloacae has been well reported. Exemplifying this is a study by Banerjee et al. in which they reported that Bacillus megaterium had very high Pb and Cd removal capacities of 95.25% and 64.17% respectively. Also, Irawati and Tahya reported that Enterobacter cloacae strains isolated from the Sukolilo River in Indonesia were able to bioaccumulate copper and had an average biosorption ability of 68% [59].
Conclusion
Summarily, this study examined the various physicochemical parameters and heavy metal concentrations of soil and water samples from Ilesha gold mining sites. HM-resistant bacteria were also isolated from the samples and were subsequently subjected to a MIC test to ascertain their resistance levels to various heavy metals including Pb, Co, Cd, Zn, and Hg while the molecular characterization of the isolated bacteria was performed. Molecular characterization revealed Priestia aryabhattai and Enterobacter cloacae as the isolates capable of resisting the high concentration of heavy metals. To the best of our knowledge, this study is the first in which the ability of Priestia aryabhattai to resist varying concentrations of Pb, Co, Cd, Zn, and Hg was explored and is also the first to report the presence of heavy metal-resisting Enterobacter cloacae in the Ilesha gold mining site. Conclusively, the identified bacteria in this study are worthy of exploration in future attempts aimed at bioremediating heavy metals in gold mining sites and other relevant applications.
Availability of data and materials
Not applicable.
Abbreviations
- HMs:
-
Heavy metals
- Co:
-
Cobalt
- Zn:
-
Zinc
- Cd:
-
Cadmium
- Pb:
-
Lead
- Hg:
-
Mercury
- Cu:
-
Copper
- Ni:
-
Nickel
- Mn:
-
Manganese
- Fe:
-
Iron
- HMRGs:
-
Heavy metal-resistance gene
- MIC:
-
Minimum inhibitory concentration
- BOD:
-
Biochemical oxygen demand
- AAS:
-
Atomic absorption spectrometer
- PCR:
-
Polymerase chain reaction
- PO4 3 :
-
Phosphate
- SO4 2 :
-
Sulphate
- NO3 − :
-
Nitrate
- HCO3 − :
-
Bicarbonate
- Cl− :
-
Chloride
- Na+ :
-
Sodium
- K+ :
-
Potassium
- Mg2+ :
-
Magnesium
- Ca2+ :
-
Calcium
- WHO:
-
World Health Organization
- EC:
-
Electrical conductivity
- ANOVA:
-
Analysis of variance
- SPSS:
-
Statistical Package for Social Sciences
References
Agboola O et al (2020) A review on the impact of mining operation: monitoring, assessment and management. Results Eng 8:100181. https://doi.org/10.1016/j.rineng.2020.100181
Khobragade K (2020) Impact of mining activity on environment: an overview. Int J Sci Res Publ 10:784–791. https://doi.org/10.29322/IJSRP.10.05.2020.p10191
Se A, Bo O, Fa O, Akinnifesi O (2018) Assessment of heavy metal pollution in a gold mining site in Southwestern Nigeria. Biomed J Sci Tech Res 12. https://doi.org/10.26717/BJSTR.2019.12.002276
Fashola M, Ngole-Jeme V, Babalola O (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047. https://doi.org/10.3390/ijerph13111047
Koller M, Saleh HM (2018) Introductory chapter: introducing heavy metals, in Heavy Metals, InTech https://doi.org/10.5772/intechopen.74783
Johnson H, Cho H, Choudhary M (2019) Bacterial heavy metal resistance genes and bioremediation potential. Comput Mol Biosci 09(01):1–12. https://doi.org/10.4236/cmb.2019.91001
Singh Sidhu GP (2016) Heavy metal toxicity in soils: sources, remediation technologies and challenges. Adv Plants Agric Res 5:1. https://doi.org/10.15406/apar.2016.05.00166
Masindi V, Muedi KL (2018) Environmental contamination by heavy metals, in Heavy Metals, InTech. https://doi.org/10.5772/intechopen.76082
M. Pichhode and Nikhil Kumar, Effect of heavy metals on plants: an overview. Int J Appl Innov Eng Manage. https://doi.org/10.13140/RG.2.2.27583.87204.
Collin S et al (2022) Bioaccumulation of lead (Pb) and its effects in plants: a review. J Hazard Mater Lett 3:100064. https://doi.org/10.1016/j.hazl.2022.100064
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782. https://doi.org/10.3390/ijerph17113782
Mitra S et al (2022) Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 34(3):101865. https://doi.org/10.1016/j.jksus.2022.101865
EL-Hak HNG, Ghobashy MA, Mansour FA, El-Shenawy NS, El-Din MIS (2022) Heavy metals and parasitological infection associated with oxidative stress and histopathological alteration in the Clarias gariepinus. Ecotoxicology. 31(7):1096–1110. https://doi.org/10.1007/s10646-022-02569-9.
Musilova J, Arvay J, Vollmannova A, Toth T, Tomas J (2016) Environmental contamination by heavy metals in region with previous mining activity. Bull Environ Contam Toxicol 97(4):569–575. https://doi.org/10.1007/s00128-016-1907-3
W. Bazzi et al (2020) Heavy metal toxicity in armed conflicts potentiates AMR in A. baumannii by Selecting for Antibiotic and Heavy Metal Co-resistance Mechanisms, Front Microbiol. 11. https://doi.org/10.3389/fmicb.2020.00068.
Yang S et al (2020) Presence of heavy metal resistance genes in Escherichia coli and Salmonella isolates and analysis of resistance gene structure in E. coli E308. J Glob Antimicrob Resist 21:420–426. https://doi.org/10.1016/j.jgar.2020.01.009
E. Terzi̇ and F. Ci̇velek, Cu, Cd, As and Hg resistance levels in Escherichia coli isolated from Mediterranean mussel and sea snail in the Southeastern Black Sea. Mar Sci Technol Bull. 2020. https://doi.org/10.33714/masteb.776902.
(2007) Report of Nigeria’s National Population Commission on the 2006 Census. Popul Dev Rev. 33(1):206–210. Available: http://www.jstor.org/stable/25434601
“BOD - Biochemical Oxygen Demand (Principle, Procedure & Application) - BiokiMicroki.” https://biokimicroki.com/bod-biochemical-oxygen-demand/. Accessed 09 May 2023
Banerjee P, Prasad B (2020) Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl Water Sci 10(5):113. https://doi.org/10.1007/s13201-020-01188-1
Kalambe NA (2021) “Determination of nitrogen in soil samples of Tiwasa Region in Amravati District.” International Virtual Conference on Materials and Nanotechnology In Association with International Journal of Scientific Research in Science and Technology 9. https://doi.org/10.32628/IJSRST221119
Abiya E, Bridget OO, Foluso O, Olufemi AJ, Akadiri S (2018) Assessment of heavy metal pollution in a gold mining site in Southwestern Nigeria. J Genet Cell Biol
A. Efetobor, F. Ologundudu, and O. Joseph Akinnifesi, Assessment of heavy metal pollution in a gold mining site in Southwestern Nigeria antimicrobial potentials view project mycology view project assessment of heavy metal pollution in a gold mining site in Southwestern Nigeria, JGCB, 2019. Available: www.scitcentral.com
3111 metals by flame atomic absorption spectrometry, in Standard Methods For the Examination of Water and Wastewater, in Standard Methods for the Examination of Water and Wastewater. Am Public Health Assoc, 2017.https://doi.org/10.2105/SMWW.2882.043
Prashanthi R, Shreevatsa GK, Krupalini S, Manoj L (2021) Isolation, characterization, and molecular identification of soil bacteria showing antibacterial activity against human pathogenic bacteria. J Genet Eng Biotechnol 19(1):120. https://doi.org/10.1186/s43141-021-00219-x
Gowsalya A, Ponnusami V, Kr S (2014) Isolation of bacteria from soil sample for exo-polysaccharide production. Int J Chemtech Res 6:2925–2928
Ibrahim UB, Kawo AH, Yusuf I, Yahaya S (2021) Physicochemical and molecular characterization of heavy metal–tolerant bacteria isolated from soil of mining sites in Nigeria. J Genet Eng Biotechnol 19(1):152. https://doi.org/10.1186/s43141-021-00251-x
Emmanuel E (2017) Isolation, identification and characterization of some bacteria from soil samples of agbaja iron ore mining site of Kogi State. Journal of Bacteriology & Mycology: Open Access 4:3. https://doi.org/10.15406/jbmoa.2017.04.00092
Kannan MN, Badoni A, Chamoli A, Chandra Bahuguna N, Sethi S (2018) Advances in agriculture and natural sciences for sustainable agriculture (October 12 &13, 2018) isolation and characterization of bacterial isolates from agriculture field soil of Roorkee region. ~ 108 ~ J Pharmacogn Phytochem, 5:108–110. Available: www.statlab.iastate.edu/survey/SQI/
L. M. , Prescott, J. P. Harley, D. A. Klein, and J. Adidas, General Microbiology, 10th ed. New york, 2018
Clsi, M07-A9: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard—Ninth Edition, 2018. [Online]. Available: www.clsi.org
A. A. Haroun, K. Kk, I. Alhaji, Y. Magaji, and E. E. Oaikhena (2017) Evaluation of heavy metal tolerance level (MIC) and bioremediation potentials of pseudomonas aeruginosa isolated from Makera-Kakuri Industrial Drain in Kaduna, Nigeria. Eur J Exp Biol. 7
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Akinnifesi TA, Asubiojo OI, Amusan AA (2006) Effects of fungicide residues on the physico-chemical characteristics of soils of a major cocoa-producing area of Nigeria. Sci Total Environ 366(2–3):876–879. https://doi.org/10.1016/j.scitotenv.2005.09.047
Fonge BA, Nkoleka EN, Asong FZ, Ajonina SA, Che VB (2017) Heavy metal contamination in soils from a municipal landfill, surrounded by banana plantation in the eastern flank of Mount Cameroon. Afr J Biotechnol 16(25):1391–1399. https://doi.org/10.5897/AJB2016.15777
Rieuwerts JS, Thornton I, Farago ME, Ashmore MR (1998) Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chem Speciat Bioavailab 10(2):61–75. https://doi.org/10.3184/095422998782775835
A. Inobeme and M. A. Adekoya, Physicochemical parameters and heavy metals characterization of soil from Okpella Mining Area in Edo State, Nigeria, 2019. [Online]. Available: https://www.researchgate.net/publication/338555946
Rakotondrabe F et al (2017) Assessment of surface water quality of Bétaré-Oya Gold Mining Area (East-Cameroon). J Water Resour Prot 09(08):960–984. https://doi.org/10.4236/jwarp.2017.98064
O. Bozorg-Haddad, M. Delpasand, and H. A. Loáiciga, Water quality, hygiene, and health, in Economical, Political, and Social Issues in Water Resources, Elsevier, 2021, pp. 217–257. doi: https://doi.org/10.1016/B978-0-323-90567-1.00008-5
Yadav APS, Dwivedi V, Kumar S, Kushwaha A, Goswami L, Reddy BS (2020) Cyanobacterial extracellular polymeric substances for heavy metal removal: a mini review. J Compos Sci 5(1):1. https://doi.org/10.3390/jcs5010001
Bandyopadhyay S, Maiti SK (2022) Steering restoration of coal mining degraded ecosystem to achieve sustainable development goal-13 (climate action): United Nations decade of ecosystem restoration (2021–2030). Environ Sci Pollut Res 29(59):88383–88409. https://doi.org/10.1007/s11356-022-23699-x
Li J, Li F, Liu Q, Zhang Y (2014) Trace metal in surface water and groundwater and its transfer in a Yellow River alluvial fan: Evidence from isotopes and hydrochemistry”. Sci Total Environ 472:979–988. https://doi.org/10.1016/j.scitotenv.2013.11.120
Ahmadou I et al (2022) Institue of Geological and Mining Research, Cameroon) 5 Biboko Area (betare-oya Gold District), East-Cameroon. J Geosci Geomatics 10(2):74–80. https://doi.org/10.12691/jgg-10-2-1
Abdu N, Yusuf AA (2013) Human health risk characterization of lead pollution in contaminated farmlands of Abare village, Zamfara State, Nigeria. 7(9):911–916. https://doi.org/10.5897/AJEST2013.1541
Udiba UU, Akpan ER (2019) Antai EE (2019) Soil lead concentrations in Dareta Village, Zamfara, Nigeria. J Health Pollut 9(23):190910. https://doi.org/10.5696/2156-9614-9.23.190910
Neethu CS, Mujeeb Rahiman KM, Saramma AV, Mohamed Hatha AA (2015) Heavy-metal resistance in Gram-negative bacteria isolated from Kongsfjord, Arctic. Can J Microbiol 61(6):429–435. https://doi.org/10.1139/cjm-2014-0803
Rahman Z, Singh VP (2018) Assessment of heavy metal contamination and Hg-resistant bacteria in surface water from different regions of Delhi, India. Saudi J Biol Sci 25(8):1687–1695. https://doi.org/10.1016/j.sjbs.2016.09.018
Ajsuvakova OP et al (2020) Sulfhydryl groups as targets of mercury toxicity. Coord Chem Rev, 417. https://doi.org/10.1016/j.ccr.2020.213343.
Zeyaullah M, Islam B, Ali A (2010) Isolation, identification and PCR amplification of merA gene from highly mercury polluted Yamuna river. Afr J Biotechnol. 9(24):3510–3514. Available: http://www.academicjournals.org/AJB
Mathivanan K, Chandirika JU, Vinothkanna A, Yin H, Liu X, Meng D (2021) Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment – a review. Ecotoxicol Environ Saf 226:112863. https://doi.org/10.1016/j.ecoenv.2021.112863
Gupta RS, Patel S, Saini N, Chen S (2020) Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int J Syst Evol Microbiol 70(11):5753–5798. https://doi.org/10.1099/ijsem.0.004475
Singh N et al (2016) Arsenic mediated modifications in Bacillus aryabhattai and their biotechnological applications for arsenic bioremediation. Chemosphere 164:524–534. https://doi.org/10.1016/j.chemosphere.2016.08.119
Joshi DN, Flora SJS, Kalia K (2009) Bacillus sp. strain DJ-1, potent arsenic hypertolerant bacterium isolated from the industrial effluent of India. J Hazard Mater 166(2–3):1500–1505. https://doi.org/10.1016/j.jhazmat.2008.12.127
Mukhopadhyay R (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26(3):311–325. https://doi.org/10.1016/S0168-6445(02)00112-2
Chang J-S, Kim Y-H, Kim K-W (2008) The ars genotype characterization of arsenic-resistant bacteria from arsenic-contaminated gold–silver mines in the Republic of Korea. Appl Microbiol Biotechnol 80(1):155–165. https://doi.org/10.1007/s00253-008-1524-0
Wu J, Rosen BP (1991) The ArsR protein is a trans-acting regulatory protein. Mol Microbiol 5(6):1331–1336. https://doi.org/10.1111/j.1365-2958.1991.tb00779.x
Singh VP, Bali A, Singh N, Jaggi AS (2014) Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 18(1):1. https://doi.org/10.4196/kjpp.2014.18.1.1
Elarabi NI et al (2020) Bacillus aryabhattai FACU: a promising bacterial strain capable of manipulate the glyphosate herbicide residues. Saudi J Biol Sci 27(9):2207–2214. https://doi.org/10.1016/j.sjbs.2020.06.050
Irawati W, Tahya CY (2021) Copper removal by Enterobacter cloacae strain IrSuk1, Enterobacter cloacae strain IrSuk4a, and Serratia nematodiphila strain IrSuk13 Isolated from Sukolilo River-Indonesia. IOP Conf Ser Mater Sci Eng 1053(1):012038. https://doi.org/10.1088/1757-899X/1053/1/012038
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: OSO and GJO. Laboratory work: AOM, GJO, OSO, BFO. Writing: GJO, VO, BFO, TO. Review: AIF, VO, and TO. Supervision: OSO and AOM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojo, G.J., Onile, O.S., Momoh, A.O. et al. Physiochemical analyses and molecular characterization of heavy metal-resistant bacteria from Ilesha gold mining sites in Nigeria. J Genet Eng Biotechnol 21, 172 (2023). https://doi.org/10.1186/s43141-023-00607-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-023-00607-5