Abstract
Background
Our aim is to evaluate LI-RADS-TR algorithm and its ability to assess the viability of TACE-treated HCC. We prospectively evaluated 100 patients with known HCC, treated with TACE and came for follow-up to assess therapy response and to plan the next step in treatment using triphasic CT study. Imaging response was evaluated according to LI-RADS-TR algorithm and compared to modified Response Evaluation Criteria in Solid Tumors (mRECIST) arterial phase hyperenhancement (APHE) criterion. Reference standard for “viable” tumors in treated observations included presence of strong tumor hyperenhancement in arterial phase and washout in the delayed phase which also shows dense accumulation of iodized oil in the target lesion.
Results
When equivocal observations were considered as LR-TR viable, LR-TR viable resulted in 92.31% sensitivity, 83.33% specificity and 88% accuracy. On the other side when equivocal observations were considered as LR-TR nonviable, it resulted in 84.62% while the specificity increased to 100% with increased accuracy (92%). The mRECIST criteria for viable tumors (presence of APHE) showed sensitivity of 84.62% and specificity of 75%. mRECIST and LR-TR sensitivities were the same when equivocal lesions were considered as nonviable and lower mRECIST than LR-TR when equivocal lesions were considered as viable, while specificities were higher in LR-TR viable being 100% when equivocal lesions were considered as nonviable, 83.33% when equivocal lesions were considered as viable and 75% in mRECIST-viable.
Conclusions
LR-TR algorithm showed good diagnostic performance compared to mRECIST, with high specificity and sensitivity when equivocal lesions were considered as nonviable, as well as improved accuracy.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the fourth most common cause of cancer-related death in the world [1]. The leading risk factor for hepatocellular carcinoma is cirrhosis due to hepatitis B or hepatitis C; consequently, the global epidemiology of HCC is determined by prevalence of dominant viral hepatitis and the age it is acquired in the underlying population. Risk factors include obesity, diabetes and related non-alcoholic fatty liver disease [2].
Treatment of HCC has been conventionally divided into curative treatment and palliative treatment. Curative treatments, such as resection and liver transplantation [3].
General agreement about a common treatment strategy for patients with HCC has not been achieved worldwide. Radical therapies, including resection, liver transplantation and percutaneous ablation (percutaneous ethanol injection (PEI) and radiofrequency (RF)), are applicable in only 30–40% of patients with HCC [4].
Also, after curative resection, recurrence is common and is the main cause of death. So, most patients are suitable only for receiving palliative treatments, such as trans-arterial chemoembolization [4].
Radiologists play a central role in the assessment of response to as trans-arterial chemoembolization for hepatocellular carcinoma. The identification of tumor viability post-treatment guides further management and potentially affects transplantation eligibility. Liver Imaging Reporting and Data Systems in 2014 introduced the concept LR- treated and a new treatment response algorithm is included in the 2017 update to assist radiologist in interpretation of response of HCC to loco-regional therapies. In addition to offering imaging criteria for viable and nonviable HCC, new concepts are introduced like non-evaluable and equivocal. The new LI-RADS treatment response algorithm offers a comprehensive approach to assess treatment response for individual lesions after a variety of TACE, contrast-enhanced CT [5].
The mRECIST for HCC has introduced the following amendments to RECIST in the determination of tumor response for target lesions. (1) Complete response: the disappearance of any intra-tumoral arterial enhancement in all target lesions; (2) Partial response: at least a 30% decrease in the sum of diameters of viable (contrast enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions; (3) Progressive disease: an increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since the treatment started; and (4) Stable disease: any cases that do not qualify for either partial response or progressive disease [6].
LI-RADS version 2017 introduces a new algorithm to standardize the reporting of treated observations, regardless of their pretreatment LI-RADS category, and is applicable after any loco-regional therapy. (1) LR-TR Non-evaluable: the treatment response cannot be evaluated due to poor image quality or inadequate technique (e.g., failure to obtain the required phases); (2) LR-TR Nonviable: this category should be assigned to treated lesions with no perceived enhancement or demonstrating only expected post-treatment enhancement patterns; (3) LR-TR Equivocal: this category is applied to treated observations that cannot be confidently categorized as viable or nonviable due to overlapping enhancement features in the absence of technical or patient-related limitations and (4) LR-TR Viable: it should be assigned to treated observations with nodular, mass-like, or thick irregular regions of arterial phase hyperenhancement (APHE), washout appearance, or enhancement similar to pretreatment tumor. These features indicate the presence of viable tumor cells with high certainty [7].
The LR-TR algorithm and the mRECIST both aim at assessing the viable tumor; however, the imaging criteria of the LR-TR algorithm include washout appearance and enhancement similar to those before treatment in addition to arterial phase hyperenhancement (APHE) [8].
Our aim was to evaluate the value of LI-RADS treatment response algorithm in the proper assessment of HCC viability using triphasic CT.
Methods
Patients
This is a prospective study that was approved by the research ethics committee of the Radiology department in our institute. All patients included in this study gave a written informed consent to participate in the research and to publish the research.
During one-year duration from July 2020 to August 2021, we prospectively evaluated 100 patients with HCC treated by TACE. Multiphasic CT examinations were done in Radiology department 1 month and 6 months after therapy. We evaluated 72 males and 28 females; the patients’ age ranged from 51 to 79 years old.
Patients with poor renal function, more than one hepatic focal lesion and patient who had TACE therapy for liver metastasis, were excluded from the study.
Alphafetoprotein levels were revised before TACE, during the first and second follow-up.
CT examination
The examinations were performed using GE light speed VCT 64 multislice CT scanner.
Non-enhanced spiral scanning was performed. Patients were then injected with non-ionic contrast material (Ultravist 370; Bayer Schering Pharma, Berlin, Germany) with peripheral venous access at a rate of 3.0 mL/s. A total of 90–120 mL (1.5 mL per kg of body weight) was injected by using a CT-compatible power injector.
Scans were acquired during the late hepatic arterial phase, portal venous phase and delayed phase. The scanning delay for late hepatic arterial phase imaging was determined using automated scan triggering software by G.E Healthcare. Arterial phase scan automatically began 10–15 s after the trigger attenuation threshold (100 HU) was reached at the level of the supra-celiac abdominal aorta. The hepatic portal venous phase scan began 30 s after the arterial phase scan. A delayed phase was performed 2–5 min after arterial phase scanning.
Image analysis
Assessed CT features included nodular, mass-like, or thick irregular APHE in or along the treated lesion, with washout appearance on PVP and/or delayed phase.
A radiologist with 5 years of experience with abdominal imaging reviewed the angiographic studies for viable target lesions, and another radiologist with 8 years of experience with abdominal imaging evaluated CT features and determined the LR-TR category for each observation after TACE and mRECIST category for the first follow-up.
For each observation, the reviewers were asked to assign the TR category (TR nonviable, TR equivocal, or TR viable) according to LR-TR algorithm.
If APHE was present in or around the lesion, it was considered mRECIST-viable, otherwise mRECIST-nonviable. While mRECIST primarily aims to determine overall disease status per patient, this study only adopted the mRECIST principle for per-lesion basis interpretation of tumor viability [9].
Tumor response according to mRECIST was calculated according to a maximum unidimensional measurement of the viable part, excluding the necrotic part.
Standard of reference
Pre-treatment CT studies were reviewed. The patients with lesions that were considered nonviable had follow-up within 6 months from the initial CT, while patient patients with lesions that were considered as viable were referred to the intervention for further treatment.
Reference standard for “viable” tumors in treated observations included presence of well-defined strong tumor hyperenhancement and delayed washout on triphasic hepatic multislice CT following TACE (3–4 weeks from TACE).
On the other hand, reference standard for “nonviable” tumors included stable or decreased lesion size on the follow-up triphasic CT (≥ 6 months from the initial follow-up) without any additional treatment.
Statistical analysis
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 26 (IBM Corp., Armonk, NY, USA). Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Standard diagnostic indices including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic efficacy were calculated as described by Galen [10]. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5 [11]. P value less than 0.05 was considered as statistically significant.
Results
Lesions
This retrospective study included 100 patients. There were 72 males and 28 females, with the patients’ age ranging from 51 to 79 years old (mean age 64 years).
Tumor response
In the post-treatment evaluation according to our gold standard, 52 observation were viable and 48 were nonviable. According to LR-TR algorithm, 44 observations were assigned as viable, 12 as equivocal and 44 were nonviable. All viable observations showed typical arterial phase hyperenhancement and delayed washout except for one observation that showed atypical arterial enhancement (regular thin marginal arterial phase enhancement) but still showed delayed washout. 16 out of 44 nonviable lesions showed good response enhancement pattern. The twelve equivocal lesions showed atypical arterial enhancement with no delayed washout. From all observations, 56 lesions were positive for mRECIST APHE criteria and 44 were negative.
Diagnostic performances of the LR-TR algorithm
When equivocal observations were considered as LR-TR viable, it resulted in sensitivity of 92.3%, specificity of 83.33% and 88% accuracy (Table 1). On the other side when equivocal observations were considered as LR-TR nonviable (Table 2), the sensitivity decreased to 84.62%, while the specificity increased to 100% with improved accuracy to 92.00%.
Comparison of performances of the LR-TR algorithm and mRECIST
The mRECIST criteria for viable tumors (presence of APHE) showed sensitivity of 84.62% and specificity of 75%, when equivocal lesions were considered as viable or nonviable. While using LR-TR, specificities were higher when equivocal lesions were considered as nonviable (100%) and decreased when equivocal lesions were considered as viable (83.33%) (Table 3).
Therapy response in the second follow-up (Table 4)
In the second follow-up after 6 months, regarding the twelve LR-TR equivocal lesions (all received treatment), eight became LR-TR nonviable and four showed arterial phase hyperenhancement with delayed washout (LR-TR viable). From the 44 viable observations (after second session of TACE), still there were 16 viable lesions, 8 became equivocal and 20 became nonviable. All viable lesions showed arterial phase hyperenhancement and delayed washout. The number of nonviable lesions that showed good response increased to 76 in the second follow-up after 6 months.
Discussion
Computed tomography (CT) plays critical role for assessing treatment response, and it is usually performed at regular intervals after TACE therapy. The goal of post-treatment imaging is to recognize residual or recurrent tumor requiring further treatment, identify complications of therapy, and detect and characterize new or additional observations elsewhere in the liver [9]. This retrospective study demonstrated good performance of the LR-TR algorithm. According to our study, the viable category of the LR-TR criteria (when LR-TR equivocal observation was considered as nonviable) showed high sensitivity (92.31%) for detecting viable tumors (Fig. 1). However, the sensitivity was 66.7% in Kim et al. [9] study and was 77% in Chaudhry et al. [12], given that the LR-TR category was determined by either the presence of APHE or washout appearance on the PVP. This discrepancy may be due variability in imaging modality or type of intervention. However, as for APHE, our study demonstrated that mRECIST criteria (presence of APHE per lesion) resulted in sensitivity of 84.62% which is less consistent with Kim et al. [9] study in which the sensitivity ranged from 92.9 to 94.0%. Thus, the higher sensitivity of LR-TR viable as compared to mRECIST indicates that the reviewers detected APHE and assign them as LR-TR viable. Unlike mRECIST, the LR-TR algorithm considers the radiologists' certainty for tumor viability which may increase the specificity by reducing false positive diagnosis in treatment-related changes such as peri-lesional hyperemia.
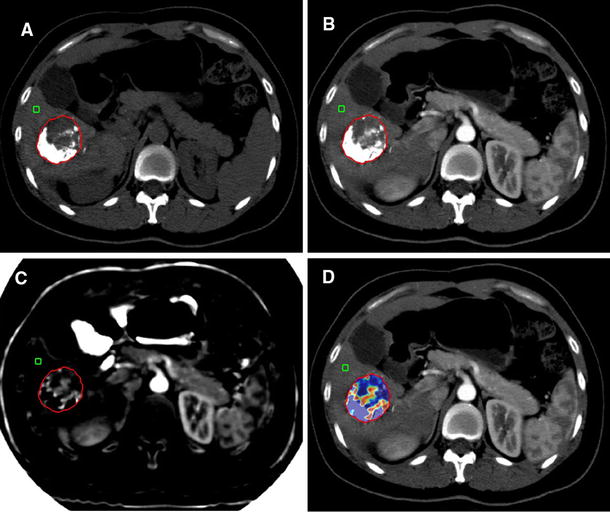
68-year-old male patient underwent TACE for right hepatic lobe segment V focal lesion. a Follow-up after one month: reveals Cirrhotic liver with right hepatic lobe segment V focal lesion, showing few peripheral lipiodol droplets with adjacent area of arterial enhancement that measures about 3 cm in maximum axial dimension with delayed washout. LR-TR category: LR-TR viable. The patient was scheduled for another session of TACE. b follow-up after second intervention: dense lipiodol packing of the previously noted segment V focal lesion with no pathological arterial enhancement or washout in the delayed phases. LR-TR category: LR-TR nonviable
The LR-TR viable category resulted in a specificity of 83.33% in our study, which was significantly higher than that for the mRECIST criteria (75%) which is also consistent with Kim et al. [9] results that were 98% for LR-TR viable category and (62.6–73.7%) for mRECIST criteria. LR-TR algorithm avoids false positive diagnosis of treatment-related changes. In clinical practice, high specificity of viable tumors is important to avoid unnecessary therapy.
Also our results yielded 100% LR-TR specificity (when equivocal observation was included with nonviable); similar to other earlier studies which were it was 97% in Chaudhry et al. [12], 99% in Cools et al. [13] and 87.9, 97.7% for CT in Seo et al. [14].
After adjusting the cutoff point for detection of a residual tumor to include LR-TR equivocal with LR-TR viable, the overall sensitivity increased to 92.31% compared to 84.62% and specificity decreased to 83.33%compared to 100%, which is quite the same concept in the study of Cools et al. [13] in which sensitivity increased to be 44% compared to 30% and specificity decreased to be 86% compared to 99%. The difference in sensitivity between our study and Cools et al. [13] was likely due to Cools et al. [13] focusing on small lesions less than 2 cm.
Also Seo et al. [14] showed great difference in sensitivity which was 54% for LR-TR viable category while assigning LR-TR equivocal as nonviable and 35% for mRECIST. This difference is mainly due to difference in reference standards used for determining tumor viability.
The negative predictive value for residual tumor was 85.71% and the positive predictive value for residual tumor was 100% when LR-TR equivocal was assigned as nonviable which is quite different with Chaudhry et al. [12] that showed negative predictive value 89–90% and positive predictive value 70–87%, but keeping with Shropshire et al. [15], which had negative predictive value of 81–87% and positive predictive value of 86–96%. These differences could be related to the difference in treatment modality, years of readers experience or imaging modality.
Our study had similar results to the study conducted by Kim et al. [9] which used clinical imaging-based diagnosis as a reference standard, and on the other side, the other studies used explanted histopathology as reference standard. Therefore, our study results may not be directly applied to predict pathologic complete response. However, considering that the LR-TR algorithm essentially aims to assess gross viable tumors, not histological viability. Kim et al. [9] believed that that study population may be more appropriate for the validation of the LR-TR criteria in general setting of post-treatment surveillance.
It should be mentioned that the follow-up studies showed disappearance of the post-treatment changes like ring of hyperemia, intra-lesional gas bubbles and central necrosis as well as reduction in size of the ablated lesions within 6 months in all nonviable lesions (Fig. 2).
a, b 61-year-old female patient underwent TACE for left hepatic lobe segment III/IVb focal lesion. a Follow-up after one month: Cirrhotic liver with left hepatic lobe segment III/IVb focal lesion showing dense lipiodol droplet with underlying no pathological enhancement. LR-TR category: LR-TR nonviable. b Follow-up after 6 months: Dense lipiodol droplet of the previously noted segment III/IVb focal lesion with no underlying pathological enhancement. LR-TR category: LR-TR nonviable
Regarding to alphafetoprotein (AFP) levels, our study showed that 100% of the patients with viable lesions (20 lesions) had persistent high levels in the second follow-up, the 8 patients with persistent equivocal lesions in the second follow-up had concomitant high alphafetoprotein levels.
Of the 76 patients with nonviable lesions in the second follow-up, the 8 patients with previously equivocal lesions and 15 of the patients with previously viable lesions showed slight decrease in the alphafetoprotein levels, while 5 showed persistent high levels (Fig. 3).
56-year-old male patient underwent TACE for right hepatic lobe segment VI focal lesion. a Follow-up after one month: Cirrhotic liver with right hepatic lobe segment VI focal lesion showing dense peripheral lipiodol droplets with adjacent area of arterial enhancement (arrow) measuring 4.3 cm in maximum axial dimension and delayed washout (arrowhead). LR-TR category: LR-TR viable. The patient was scheduled for another session of TACE. b Follow-up after second intervention: Cirrhotic liver with segment VI focal lesion showing slight progression in size of the previously noted arterially enhancing area (arrow) measuring 4.5 cm maximum axial dimension with washout in the delayed phase (arrowhead). LR-TR category: LR-TR viable
While 30 patients of 48 with nonviable lesions had significant drop in AFP level, 18 showed slight decrease in its level.
Our study had few limitations. According to Park et al. [8], first, the lack of histological proof, which can lead to overestimated response. Thus, further studies including a pathological gold standard would be of great value to correlate the response and the histological tumor grade in limiting inclusion to pre-transplantation or surgery cases. Second, the high density of lipiodol material in CT which poses difficulty in the proper detection of tumor viability, so dynamic MRI would be recommended to avoid such limitation. In some institutions, MRI is the golden standard for post-TACE assessment of HCC.
Conclusions
In conclusion, the LR-TR algorithm showed good diagnostic performance compared to mRECIST by triphasic CT, with high specificity and sensitivity which increased when equivocal lesions were considered as nonviable, with improved accuracy.
Availability of data and materials
All the datasets used and analyzed during this study are available with the corresponding author on reasonable request.
Abbreviations
- LR-TR:
-
Liver imaging reporting and data system treatment response algorithm
- mRECIST:
-
Modified response evaluation criteria in solid tumors
- TACE:
-
Trans-catheter arterial chemoembolization
- APHE:
-
Arterial phase hyperenhancement
- CI:
-
Confidence interval
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Mittal S, El-Serag HB (2013) Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 47:S2–S6. https://doi.org/10.1097/MCG.0b013e3182872f29
Granito A, Bolondi L (2009) Medical treatment of hepatocellular carcinoma. Mediterr J Hematol Infect Dis 1(3):e2009021. https://doi.org/10.4084/MJHID.2009.021
Marelli L, Stigliano R, Triantos C et al (2006) Treatment outcomes for hepatocellular carcinoma using chemoembolization in combination with other therapies. Cancer Treat Rev 32(8):594–606
Kielar A, Fowler KJ, Lewis S et al (2018) Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol 43(1):218–230. https://doi.org/10.1007/s00261-017-1281-6
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. In: Seminars in liver disease, vol 30, no 01. Thieme Medical Publishers, pp 052–060. https://doi.org/10.1055/s-0030-1247132
Voizard N, Cerny M, Assad A et al (2019) Assessment of hepatocellular carcinoma treatment response with LI-RADS: a pictorial review. Insights Imaging 10(1):1–22
Park S, Joo I, Lee DH et al (2020) Diagnostic performance of LI-RADS treatment response algorithm for hepatocellular carcinoma: adding ancillary features to MRI compared with enhancement patterns at CT and MRI. Radiology. https://doi.org/10.1148/radiol.2020192797
Kim SW, Joo L, Kim H-C et al (2020) LI-RADS treatment response categorization on gadoxetic acid-enhanced MRI: diagnostic performance compared to mRECIST and added value of ancillary features. Eur Radiol 30:2861–2870. https://doi.org/10.1007/s00330-019-06623-9
Galen RS (1980) Predictive values and efficiency of laboratory testing. Pediatr J Clin N Am 27:861–869
Chan YH (2003) Biostatistics 103: qualitative data—tests of independence. Singap Med J 44(10):498–503
Chaudhry M, McGinty KA, Mervak B et al (2019) The LI-RADS version 2018 MRI treatment response algorithm: evaluation of ablated hepatocellular carcinoma. Radiology 294:320–326
Cools KS, Moon AM, Burke LMB et al (2020) Validation of the liver imaging reporting and data system treatment response criteria after thermal ablation for hepatocellular carcinoma. Liver Transpl 26(2):203–214
Seo N, Kim MS, Park MS et al (2020) Evaluation of treatment response in hepatocellular carcinoma in the explanted liver with Liver Imaging Reporting and Data System version 2017. Eur Radiol 30(1):261–271
Shropshire EL, Chaudhry M, Miller CM et al (2019) LI-RADS treatment response algorithm: performance and diagnostic accuracy. Radiology 292:226–234. https://doi.org/10.1148/radiol.2019182135
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HE, EN, SE and MG contributed equally to this work. HE and MG designed research. EN performed research. HE, SE and EN analyzed data. HE and MG wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study way approved by the research ethics committee of the Radiology department of the Faculty of medicine Cairo University on 3/3/2020, Reference number of approval: 820-2020. All patients included in this study gave a written informed consent to participate in the research.
Consent for publication
All patients included in this study gave a written informed consent to publish the data contained in this study.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Assaly, H., Abdelwahab, E., El Sebai, S.M. et al. Can triphasic hepatic multislice CT validate the LI-RADS treatment response algorithm after trans-catheter arterial chemoembolization treatment of hepatocellular carcinoma?. Egypt J Radiol Nucl Med 54, 3 (2023). https://doi.org/10.1186/s43055-022-00939-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00939-1