Abstract
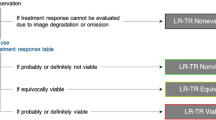
Radiologists play a central role in the assessment of patient response to locoregional therapies for hepatocellular carcinoma (HCC). The identification of viable tumor following treatment guides further management and potentially affects transplantation eligibility. Liver Imaging Reporting and Data Systems (LI-RADS) first introduced the concept of LR-treated in 2014, and a new treatment response algorithm is included in the 2017 update to assist radiologists in image interpretation of HCC after locoregional therapy. In addition to offering imaging criteria for viable and nonviable HCC, new concepts of nonevaluable tumors as well as tumors with equivocal viability are introduced. Existing guidelines provided by response evaluation criteria in solid tumors (RECIST) and modified RECIST address patient-level assessments and are routinely used in clinical trials but do not address the variable appearances following different locoregional therapies. The new LI-RADS treatment response algorithm addresses this gap and offers a comprehensive approach to assess treatment response for individual lesions after a variety of locoregional therapies, using either contrast-enhanced CT or MRI.











Similar content being viewed by others
References
Ahmed M, Solbiati L, Brace CL, et al. (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1):241–260
Cheung W, Kavnoudias H, Roberts S, et al. (2013) Irreversible electroporation for unresectable hepatocellular carcinoma: initial experience and review of safety and outcomes. Technol Cancer Res Treat 12(3):233–241
Gaba RC, Lewandowski RJ, Hickey R, et al. (2016) Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 27(4):457–473
Benedict SH, Yenice KM, Followill D, et al. (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37(8):4078–4101
Halperin EC, Perez CA, Wazer DE (2013) Perez and Brady’s principles and practice of radiation oncology, 6th edn. Philadelphia: Lippincott Williams & Wilkins
Kim SK, Lim HK, Kim YH, et al. (2003) Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics 23(1):107–121
Chung WS, Lee KH, Park MS, et al. (2012) Enhancement patterns of hepatocellular carcinoma after transarterial chemoembolization using drug-eluting beads on arterial phase CT images: a pilot retrospective study. AJR Am J Roentgenol 199(2):349–359
Yaghmai V, Besa C, Kim E, et al. (2013) Imaging assessment of hepatocellular carcinoma response to locoregional and systemic therapy. AJR Am J Roentgenol 201(1):80–96
Ehman EC, Umetsu SE, Ohliger MA, et al. (2016) Imaging prediction of residual hepatocellular carcinoma after locoregional therapy in patients undergoing liver transplantation or partial hepatectomy. Abdom Radiol 41(11):2161–2168
Kallini JR, Miller FH, Gabr A, Salem R, Lewandowski RJ (2016) Hepatic imaging following intra-arterial embolotherapy. Abdom Radiol 41(4):600–616
Furui S, Otomo K, Itai Y, Iio M (1984) Hepatocellular carcinoma treated by transcatheter arterial embolization: progress evaluated by computed tomography. Radiology 150(3):773–778
Kimura T, Takahashi S, Kenjo M, et al. (2013) Dynamic computed tomography appearance of tumor response after stereotactic body radiation therapy for hepatocellular carcinoma: how should we evaluate treatment effects? Hepatol Res 43(7):717–727
Kim EY, Choi D, Lim DH, et al. (2009) Change in contrast enhancement of HCC on 1-month follow-up CT after local radiotherapy: an early predictor of final treatment response. Eur J Radiol 72(3):440–446
Kimura T, Takahashi S, Takahashi I, et al. (2015) The time course of dynamic computed tomographic appearance of radiation injury to the cirrhotic liver following stereotactic body radiation therapy for hepatocellular carcinoma. PLoS ONE 10(6):e0125231
Park MJ, Kim SY, Yoon SM, et al. (2014) Stereotactic body radiotherapy-induced arterial hypervascularity of non-tumorous hepatic parenchyma in patients with hepatocellular carcinoma: potential pitfalls in tumor response evaluation on multiphase computed tomography. PLoS ONE 9(2):e90327
Mannelli L, Kim S, Hajdu CH, et al. (2009) Assessment of tumor necrosis of hepatocellular carcinoma after chemoembolization: diffusion-weighted and contrast-enhanced MRI with histopathologic correlation of the explanted liver. AJR Am J Roentgenol 193(4):1044–1052
Merkle EM, Zech CJ, Bartolozzi C, et al. (2016) Consensus report from the 7th international forum for liver magnetic resonance imaging. Eur Radiol 26(3):674–682
Shinagawa Y, Sakamoto K, Fujimitsu R, et al. (2010) Pseudolesion of the liver observed on gadoxetate disodium-enhanced magnetic resonance imaging obtained shortly after transarterial chemoembolization for hepatocellular carcinoma. Jpn J Radiol 28(6):483–488
Watanabe H, Kanematsu M, Goshima S, et al. (2012) Is gadoxetate disodium-enhanced MRI useful for detecting local recurrence of hepatocellular carcinoma after radiofrequency ablation therapy? AJR Am J Roentgenol 198(3):589–595
Liapi E, Geschwind JF (2012) Combination of local transcatheter arterial chemoembolization and systemic anti-angiogenic therapy for unresectable hepatocellular carcinoma. Liver Cancer 1(3–4):201–215
Weintraub JL, Salem R (2013) Treatment of hepatocellular carcinoma combining sorafenib and transarterial locoregional therapy: state of the science. J Vasc Interv Radiol 24(8):1123–1134
Chaudhury PC, Hassanain M, Bouteaud JM, et al. (2010) Complete response of hepatocellular carcinoma with sorafenib and Y radioembolization. Curr Oncol 17(5):67–69
Siegel AB, Cohen EI, Ocean A, et al. (2008) Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 26(18):2992–2998
Sun W, Sohal D, Haller DG, et al. (2011) Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 117(14):3187–3192
Llovet JM, Bruix J (2008) Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48(4):1312–1327
Llovet JM, Ricci S, Mazzaferro V, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Harding JJ, El Dika I, Abou-Alfa GK (2016) Immunotherapy in hepatocellular carcinoma: primed to make a difference? Cancer 122(3):367–377
Wolchok JD, Hoos A, O’Day S, et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15(23):7412–7420
Donati OF, Do RK, Hötker AM, et al. (2015) Interreader and inter-test agreement in assessing treatment response following transarterial embolization for hepatocellular carcinoma. Eur Radiol 25(9):2779–2788
Sato Y, Watanabe H, Sone M, et al. (2013) Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci 118(1):16–22
Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. (2015) Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology 62(4):1111–1121
Shim JH, Lee HC, Won HJ, et al. (2012) Maximum number of target lesions required to measure responses to transarterial chemoembolization using the enhancement criteria in patients with intrahepatic hepatocellular carcinoma. J Hepatol 56(2):406–411
Hunt SJ, Yu W, Weintraub J, Prince MR, Kothary N (2009) Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol 20(1):30–38
Gazelle GS, Goldberg SN, Solbiati L, Livraghi T (2000) Tumor ablation with radio-frequency energy. Radiology 217(3):633–646
Shi W, He Y, Ding W, et al. (1064) Contrast-enhanced ultrasonography used for post-treatment responses evaluation of radiofrequency ablations for hepatocellular carcinoma: a meta-analysis. Br J Radiol 2016(89):20150973
Liu M, Lin MX, Xu ZF, et al. (2015) Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol 25(8):2502–2511
Takizawa K, Numata K, Morimoto M, et al. (2013) Use of contrast-enhanced ultrasonography with a perflubutane-based contrast agent performed one day after transarterial chemoembolization for the early assessment of residual viable hepatocellular carcinoma. Eur J Radiol 82(9):1471–1480
Tsuda M, Majima K, Yamada T, et al. (2001) Hepatocellular carcinoma after radiofrequency ablation therapy: dynamic CT evaluation of treatment. Clin Imaging 25(6):409–415
Shyn PB, Oliva MR, Shah SH, et al. (2012) MRI contrast enhancement of malignant liver tumours following successful cryoablation. Eur Radiol 22(2):398–403
McLoughlin RF, Saliken JF, McKinnon G, Wiseman D, Temple W (1995) CT of the liver after cryotherapy of hepatic metastases: imaging findings. AJR Am J Roentgenol 165(2):329–332
Schima W, Ba-Ssalamah A, Kurtaran A, Schindl M, Gruenberger T (2007) Post-treatment imaging of liver tumours. Cancer Imaging 7(A):S28–S36
Wang X, Erinjeri JP, Jia X, et al. (2013) Pattern of retained contrast on immediate postprocedure computed tomography (CT) after particle embolization of liver tumors predicts subsequent treatment response. Cardiovasc Interv Radiol 36(4):1030–1038
Kubota K, Hisa N, Nishikawa T, et al. (2001) Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging 26(2):184–190
Lee S, Kim KA, Park MS, Choi SY (2015) MRI findings and prediction of time to progression of patients with hepatocellular carcinoma treated with drug-eluting bead transcatheter arterial chemoembolization. J Korean Med Sci 30(7):965–973
Ibrahim SM, Nikolaidis P, Miller FH, et al. (2009) Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom Imaging 34(5):566–581
Therasse P, Arbuck SG, Eisenhauer EA, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Galizia MS, Töre HG, Chalian H, et al. (2012) MDCT necrosis quantification in the assessment of hepatocellular carcinoma response to yttrium 90 radioembolization therapy: comparison of two-dimensional and volumetric techniques. Acad Radiol 19(1):48–54
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Acknowledgment
We thank Joanne Chin for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Ania Kielar was funded by General Electric. Richard K Do received support from the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kielar, A., Fowler, K.J., Lewis, S. et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol 43, 218–230 (2018). https://doi.org/10.1007/s00261-017-1281-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1281-6