Abstract
Background
Advances in paediatric critical care have resulted in a reduction in mortality. This has shifted the focus to paediatric intensive care unit (PICU)-related morbidities and how to reduce or prevent them. In this study, we aimed to study the impact of paediatric intensive care unit (PICU) admission on neurocognitive performance.
Methods
Intelligence quotient (IQ) was tested in 50 children (27 boys, 23 girls; mean age 6.98 years) 3 months after PICU discharge and in 75 controls using the Stanford-Binet IQ test.
Results
There was no statistically significant difference between patients and controls with regard to IQ scores, and no difference between medical and surgical patients (p > 0.05). IQ was unaffected by sedation, blood transfusion, or blood product transfusion. Patients who underwent a major surgical procedure, needed inotropic support, and needed mechanical ventilation had non-significantly lower IQ scores than those who did not. A non-significant negative correlation was observed between the length of PICU stay, mechanical ventilation duration, sedative use, and inotropic support.
Conclusions
PICU admission does not appear to significantly affect cognitive outcomes in paediatric survivors.
Similar content being viewed by others
What is already known on the topic?
-
Recent advances in paediatric critical care have dramatically improved patient survival; therefore, attention has shifted from mortality to possible morbidities.
-
Post-intensive care syndrome (PICS) is well characterised in adults and involves impairment of quality of life and cognitive and neuropsychiatric functions.
-
Similarly, PICS is thought to occur in children; studying its varied aspects is needed for the characterisation of this syndrome in this age group to allow for better prediction of long-term outcomes following admission to PICU.
What this paper adds
-
PICU admission may not be associated with adverse cognitive outcomes in children.
-
Confounding factors, including variability of patient characteristics, admission diagnoses, and pre-admission health status, are thought to affect long-term outcomes irrespective of the events that occur during PICU admission.
-
Further research is needed using standardised patient groups, clinical characteristics, type, and time of assessment methods employed before a generalizable conclusion about the impact of PICU admission on cognitive abilities in children can be made.
Background
Critical care advancement has improved outcomes following paediatric intensive care unit (PICU) admission [1]. An Egyptian tertiary hospital PICU had a mortality rate of 33.1% [2]. African and Middle Eastern countries have reported mortality rates of 30.9–37.4% between 2013 and 2016 [3,4,5]. Besides mortality, studying the extent and impact of morbidities on paediatric critical illness is crucial [6].
Research on adults with critical illness has shown several physical, neuropsychiatric, and quality of life (QoL) impairments causing permanent global and executive function defects [7]. This “post-intensive care syndrome” (PICS) is also seen in children [8].
The PICS in paediatrics (PICS-p) framework was proposed to conceptualise PICS in children by assessing potential post-PICU physical, cognitive, emotional, and social health impairments. The interaction between the pre-admission health statuses, the cause, PICU admission course, and how families handle resultant impairments are thought to determine the children’s outcomes. Studies are needed to validate and refine framework aspects to determine the outcomes of paediatric critical illness [9].
Recent paediatric studies have investigated physical [1, 10,11,12,13,14,15], neurocognitive [16,17,18], and psychological morbidities [19]. Significant study population variability and their outcome measures have caused many confounding factors [9].
We assessed the cognitive impact of PICU admission in children, after excluding patients with a disease or condition thought to independently affect cognition.
Methods
Patient recruitment and consent
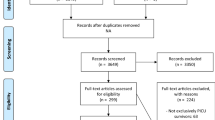
Enrolled children were over 2 years old at admission and discharged alive from our institution; their different admission diagnoses are listed in Additional file 1: Tables S1 and S2. Patient data were collected during their ICU stay with parental or legal guardians’ consent to conduct the test 3 months post-discharge.
Children with pre-existing neurological morbidity, known chronic illness, genetic syndrome, admission for neurological reasons, or with seizure development during PICU admission were excluded. We excluded likely cognition-affecting factors, irrespective of PICU events and interventions. Exclusion reasons were as follows: (1) < 24 h in the ICU, (2) parents or legal guardians refused participation, (3) re-admitted to the PICU for > 48 h post-initial discharge, and (4) no discharge summary or contact details available.
Healthy age- and gender-matched controls without known chronic illness or any parent or teacher concerns about their motor or mental development and no acute illness at the assessment time were recruited from the patients’ family and friends.
Study protocol
This prospective cohort study was conducted in a tertiary university paediatric hospital PICU that is equipped with 25 beds and serves approximately 200 patients/year. We aimed to assess the neurocognitive outcome of the PICU survivors.
Fifty children discharged from the PICU (consecutively recruited between June 2018 and December 2018) and 75 controls underwent intelligence domain neurocognitive testing using the Stanford-Binet Intelligence Scale Fifth Edition (SB-5) 3 months post-ICU discharge. The 3-month point reflecting the short-term cognitive outcome was selected. Furthermore, literature review showed that most similar studies assessed children at 3 (and maybe again at 9 or 12) months post-PICU discharge to assess short- and long-term outcomes.
The SB-5 scale assesses the total intelligence quotient (TIQ), verbal intelligence quotient (VIQ), and performance (non-verbal) intelligence quotient (PIQ). The results are further used to categorise patients and controls into five categories: average (IQ 90–109), dull average (IQ 80–89), borderline (IQ 70–79), mildly impaired (IQ 55–69), and impaired (IQ 40–54) [20].
The scale diagnoses many developmental disabilities and is commonly used for paediatric cognitive assessment (age ≥ 2 years). It has a strong correlation with other intelligence tests and is the most used test according to the 2001 US Census [20].
We chose the SB5 as an intelligence test because (1) of reliability and validity evidence; (2) it covers the widest age range using a single test, with easy administration and data collection and analysis; and (3) we could enrol children as young as 2 years old, and this was not an option with other tests. The last point is important, as many of our PICU children were in the younger age groups.
Statistical analyses (using SPSS version 25.0; IBM Corp., Armonk, NY) were performed according to each parameter’s data type, using descriptive (mean, standard deviation, parametric numerical data range, non-numerical data frequency, and percentage) and analytical statistics. Student’s t, chi-square, and Fisher’s exact tests were used for statistical significance difference assessment between the two study groups and to study the relationship between two qualitative variables when the expected count was < 5 in over 20% of the cells, respectively. Correlation analysis (using Pearson’s method) was used to assess the strength of the association between two quantitative variables. The correlation coefficient denoted r defines the strength and direction of the linear relationship between two variables (r = 0–0.19, very weak correlation; r = 0.2–0.39, weak correlation; r = 0.40–0.59, moderate correlation; r = 0.6–0.79, strong correlation; and r = 0.8–1, very strong correlation). The p-value was used to determine significance (p > 0.05, non-significant; p < 0.05, significant; and p < 0.01, highly significant).
Results
The patients’ mean age was 6.08 ± 4.05 years (range: 2–16, 54% [27/50] male). The admission rate was 38% for medical reasons and 62% for postoperative management. Among these, 54% needed sedation, 38% assisted ventilation, and 12% required inotropic support. Only 8% required blood or blood product transfusions. The mean PICU length of stay (LOS) was 5.9 ± 2.5 days.
Most surgical patients underwent major operations (85.2%). (Additional file 1: Table S2). Postoperative complications, such as sepsis and wound infection, were recorded in 16% of the patients.
Patients and controls had similar IQ (p > 0.05) (Table 1), with no difference between male and female patients (p > 0.05) and between medical and surgical patients (p > 0.05). However, 6% of the surgical patients had borderline and mildly impaired IQ (Table 2).
The mean verbal, performance, and total IQ values were lower in the major surgery group, though the difference was statistically insignificant (p-values VIQ: 0.12, PIQ: 0.20, and TIQ: 0.11). Among the major surgery group, 8.7% were in the mildly impaired and borderline IQ category and 17% were in the dull average category, whereas 100% of the minor surgery group fell in the average IQ category (Table 2).
Surgical patients with and without postoperative complications were statistically similar. The mean IQ subset values and the patient percentages in the different IQ categories were also comparable (Table 2).
The mean verbal, performance, and total IQ values were lower in the assisted ventilation group than in the group without assisted ventilation (p-value = 0.058). Moreover, children who needed assisted ventilation were in the lower total IQ categories (p-value = 0.057). The mean verbal, performance, and total IQ scores were lower in the group that needed inotropic support during their ICU stay, although statistically insignificant (Table 2).
Sedative use and blood transfusion were not associated with a significant IQ difference. There was a consistent negative correlation between verbal, performance, and total IQ scores and PICU LOS, mechanical ventilation, sedative use, and inotropic support, though statistically insignificant (Table 3).
Discussion
Studies that have evaluated the PICU admission impact on health-related QoL, and cognitive abilities in children without pre-existing or concurrent neurological disorders are scarce [16,17,18]. We observed no statistically significant difference in IQ between patients and controls. Conflicting previous studies report minimal, if any deficits, after PICU admission [10, 11, 13, 14] or a substantial negative impact on QoL, functional outcome, and cognition [17, 21,22,23]. One study reported persistently poor academic performances post-discharge from PICU [
The heterogeneous demographic and clinical characteristics of previous PICU cohorts could have caused this variability. Our cohorts had a mean age of 6.08 years, while it ranged from 1 to 10 years in other studies [1, 11, 13, 14, 16, 17, 22]. In one study, the age range was 2.5–31.6 years [14]. Different age groups with different admission diagnoses underwent different interventions. Thus, finding a unified assessment approach is difficult.
Different assessment tools, including the Multi-Attribute Health Status Classification System [10, 11], Health State Utilities Index [12, 13, 15], generic questionnaires [13], the Paediatric Cerebral Performance Category for cognitive morbidity, the Paediatric Overall Performance Category [22], and various neuropsychological batteries assessing multiple cognitive domains and academic performance, were used across the variable cohorts [16, 17]. This lack of assessment method standardisation makes outcome comparison difficult [9, 16].
School performance or the ability to live independently may not always be appropriate to assess very young children incapable of either [1, 10, 11]. Several current assessment methods have not been validated in infants, who constitute a substantial percentage of PICU admissions.
As in our cohort, a slight male preponderance was observed in most paediatric PICU studies (54–65%) [12, 13, 16, 18, 22]. Our medical patients constituted only 38% of the cohort, and the rest were surgical patients. In one study, surgical patients outnumbered medical patients [10]. In other studies, the opposite was observed [20].
Mechanical ventilation and inotropic support were needed in 38% and 12% of our patients, respectively. The need for both varied widely between studies (30–76% mechanical ventilation [1, 12,13,14] and 27–40.6% inotropic support [1, 13, 14]).
We observed a consistent, non-significant negative correlation between IQ values and the duration of mechanical ventilation, sedation, and inotropic support. Mechanical ventilation and sedation are independent risk factors for cognitive impairment post-PICU discharge [23]. While both have side effects, children requiring more aggressive intervention in the PICU probably had a pre-existing severe illness, and this is probably the more important determinant of functional outcome.
The mean length of PICU stay here was 5.9 ± 2.5 days. The range in similar studies was 4.24–5.7 days [10, 11, 13, 22]. We observed that the mean LOS negatively correlated with IQ values, though insignificantly. In one study, a prolonged paediatric PICU stay had unfavourable outcomes; however, these patients had more comorbidities or disabilities than did the short-stay patients [10]. Neither illness severity at admission nor PICU LOS were risk factors for subsequent cognitive morbidity in a recent meta-analysis [23].
We excluded children with pre-existing neurological diseases that were likely to impact functional outcomes. In most similar studies, 12–66% of patients had pre-existing, common neurological morbidities [1, 11, 14, 22] that were associated with worse long-term outcomes [1, 10].
We excluded children admitted with acute neurological illnesses like meningitis (an independent poor outcome predictor). Admissions for acute neurological illnesses constitute 6–18% of different PICU cohorts [1, 14, 16, 22] with poorer outcomes [10, 11]. Such studies have specified deficits in children with septic illness, including meningococcal disease [16, 17] and patients with pre-morbid conditions [17].
In one study, seizures during admission were also a predictor of worse outcomes. Although seizures could be due to hypoxia or electrolyte disturbance that led to PICU interventions [24], they could also be symptoms of primary neurological illnesses like meningitis or sepsis with central nervous system involvement. In another review, seizures were not a risk factor for cognitive impairment post-PICU admission [23].
PICU-related morbidities tend to resolve over time [9, 13]. Therefore, a problem with previous studies is the lack of standardisation about the time point for the assessment tests [1, 10, 12,13,14,15,16,17]. Tests were carried out at varied time points in one study [14]. One study that concluded a persistent cognitive deficit over time had a high case drop-out rate and included subsets of children with sepsis, meningitis, and chronic illness but did not specify the subsets suffering from persistent neuropsychological deficits. The trend was followed by the group as a whole [18].
More patients are eligible for any study than those who participate [12, 17] and differ in many factors, including the illness severity at admission [7], non-completion of questionnaires due to language barrier [8], and gender and ethnic characteristics [17].
Our study has some limitations: (1) Pre-PICU cognitive assessment data were unavailable; these were also missing in similar studies as cognitive testing is uncommon, especially for previously healthy children. To overcome this, we used healthy age- and gender-matched controls. (2) The small number of patients prevents us from generalising our conclusions. (3) It is important to repeat cognitive testing to assess permanent deficits. (4) Infants, constituting more than half of our PICU patients, were excluded due to the lack of a readily available validated cognition test, and this could have led to the loss of important data.
Conclusion
PICU admission does not appear to be associated with adverse neurodevelopmental outcomes. Prospective long-term, large-scale studies on paediatric patients after being discharged from the PICU are needed, with cognitive assessment test and administration time point standardisation to generate more reliable results in study comparisons. With neurological problems like meningitis, encephalitis, or pre-existing neurological disease, a worse neurocognitive outcome post-PICU discharge should be cautiously interpreted. Ideally, these patients should be excluded from studies that assess neurocognitive outcomes or should be studied as a separate group. Finally, post-PICU discharge, children with residual motor or cognitive deficit should be offered appropriate, timely rehabilitation and educational interventions.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PICU:
-
Paediatric intensive care unit
- IQ:
-
Intelligence quotient
- PICS:
-
Post-intensive care syndrome
- PICS:
-
Post-intensive care syndrome – paediatric
- QoL:
-
Quality of life
- SB-5:
-
Stanford-Binet Test, 5th edition
- TIQ:
-
Total intelligence quotient
- PIQ:
-
Performance intelligence quotient
- VIQ:
-
Verbal intelligence quotient
- LOS:
-
Length of stay
References
Namachivayam P, Shann F, Shekerdemian L et al (2010) Three decades of paediatric intensive care: who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med 11:549–555
Rady HI (2014) Profile of patients admitted to paediatric intensive care unit, Cairo University Hospital: 1-year study. Ain-Shams J Anaesthesiol 7:500–503
Embu HY, Yiltok SJ, Isamade ES, Nuhu SI, Oyeniran OO, Uba FA (2011) Paediatric admissions and outcome in a general intensive care unit. Afr J Paediatr Surg 8:57–61
Alsuheel AM, Shati AA (2014) Factors predicting mortality in paediatric intensive care unit in a tertiary care center Southwest Region, Saudi Arabia. J Med Med Sci 5:113–120
Tazebew AA, Cahkilu B, Bacha T (2019) Admission pattern and outcome in a paediatric intensive care unit of Gondar University hospital. Ethiop Med J 57:2
Herrup EA, Wieczorek B, Kudchadkar SR (2017) Characteristics of post-intensive care syndrome in survivors of paediatric critical illness: a systematic review. World J Crit Care Med 6:124–134
Pandharipande PP, Girard TD, Jackson JC et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369:1306–1316
Needham DM, Davidson J, Cohen H et al (2012) Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 40:502–509
Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ (2018) Conceptualizing post intensive care syndrome in children-the PICS-p Framework. Pediatr Crit Care Med 19:298–300
Gemke RJ, Bonsel GJ, van Vught AJ (1995) Long-term survival and state of health after paediatric intensive care. Arch Dis Child 73:196–201
Jayshree M, Singhi SC, Malhi P (2003) Follow up of survival and quality of life in children after intensive care. Indian Pediatr 40:303–309
Jones S, Rantell K, Stevens K et al (2006) Outcome at 6 months after admission for paediatric intensive care: a report of a national study of paediatric intensive care units in the United Kingdom. Pediatrics 118:2101–2108
Knoester H, Bronner MB, Bos AP, Grootenhuis MA (2008) Quality of life in children three and nine months after discharge from a paediatric intensive care unit: a prospective cohort study. Health Qual Life Outcomes 6:21
Taylor A, Butt W, Ciardulli M (2003) The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med 29:795–800
Namachivayam P, Taylor A, Montague T et al (2012) Long-stay children in intensive care: long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr Crit Care Med 13:520–528
Elison S, Shears D, Nadel S, Sahakian B, Garralda ME (2008) Neuropsychological function in children following admission to paediatric intensive care: a pilot investigation. Intensive Care Med 34:1289–1293
Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME (2013) Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med 41:1094–1103
Als LC, Tennant A, Nadel S, Cooper M, Pierce CM, Garralda ME (2015) Persistence of neuropsychological deficits following paediatric critical illness. Crit Care Med 43:e312–e315
Als LC, Picouto MD, Hau SM et al (2015) Mental and physical well-being following admission to paediatric intensive care. Pediatr Crit Care Med 16:e141–e149
Roid GH (2003) Stanford-Binet Intelligence Scales, 5th edn. Riverside Publishing, Itasca
Kaufman AS (2009) IQ Testing 101. Springer Publishing, New York
Alievi PT, Carvalho PRA, Trotta EA, Filho RM (2007) Impacto da internação em unidade de terapia intensiva pediátrica: avaliação por meio de escalas de desempenho cognitivo e global. J Pediatr 83:505–511
Kachmar AG, Irving SY, Connolly CA, Curley MAQ (2018) A systematic review of risk factors associated with cognitive impairment after paediatric critical illness. Pediatr Crit Care Med 19:e164–e171
Idro R, Gwer S, Kahindi M et al (2008) The incidence, aetiology and outcome of acute seizures in children admitted to a rural Kenyan district hospital. BMC Pediatr 8:5
Acknowledgements
We would like to thank Sarah Mohamed Saeed, specialist paediatrician, for her help with data collection and Mr. Abdul Gawad Mohamed, specialist psychologist, for his help with administering the SB5 to the study participants. We would also like to thank Dr. Mostafa Yosef El-Shahed, lecturer of community medicine at Ain Shams University, for his work on the statistical analysis and review of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception and design, acquisition of the data, or analysis and interpretation of the data: R.H.A., A.R.R., and R.M.H.Z. Drafting of the article or its critical revision for important intellectual content: R.M.H.Z. Final approval of the version to be published: R.H.A., A.R.R., and R.M.H.Z. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ain Shams University Pediatric Department Council (approval number 22441).
Consent for publication
A verbal informed consent for participation was obtained from the parents of patients immediately before they were discharged from the PICU and from the parents or guardians of the healthy control subjects.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Admission diagnoses (medical patients). Table S2. Admission diagnoses (surgical patients).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaitoun, R., Aly, R.H. & Rezk, A.R. Impact of paediatric intensive care unit admission on neurocognitive function in children. Egypt Pediatric Association Gaz 70, 25 (2022). https://doi.org/10.1186/s43054-022-00114-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-022-00114-1