Abstract
Background
Long-term morbidity after pediatric intensive care unit (PICU) admission is a growing concern. Both critical illness and accompanying PICU treatments may impact neurocognitive development as assessed by its gold standard measure; intelligence. This meta-analysis and meta-regression quantifies intelligence outcome after PICU admission and explores risk factors for poor intelligence outcome.
Methods
PubMed, Embase, CINAHL and PsycINFO were searched for relevant studies, published from database inception until September 7, 2021. Using random-effects meta-analysis, we calculated the standardized mean difference in full-scale intelligence quotient (FSIQ) between PICU survivors and controls across all included studies and additionally distinguishing between PICU subgroups based on indications for admission. Relation between demographic and clinical risk factors and study’s FSIQ effect sizes was investigated using random-effects meta-regression analysis.
Results
A total of 123 articles was included, published between 1973 and 2021, including 8,119 PICU survivors and 1,757 controls. We found 0.47 SD (7.1 IQ-points) lower FSIQ scores in PICU survivors compared to controls (95%CI -0.55 to -0.40, p < .001). All studied PICU subgroups had lower FSIQ compared to controls (range 0.38–0.88 SD). Later year of PICU admission (range 1972–2016) and longer PICU stay were related to greater FSIQ impairment (R2 = 21%, 95%CI -0.021 to -0.007, p < .001 and R2 = 2%, 95%CI -0.027 to -0.002, p = .03, respectively), whereas male sex and higher rate of survivors were related to smaller FSIQ impairment (R2 = 5%, 95%CI 0.001 to 0.014, p = .03 and R2 = 11%, 95%CI 0.006 to 0.022, p < .001, respectively). Meta-regression in PICU subgroups showed that later year of PICU admission was related to greater FSIQ impairment in children admitted after cardiac surgery and heart- or heart–lung transplantation. Male sex was related to smaller FSIQ impairment in children admitted after cardiac surgery. Older age at PICU admission and older age at follow-up were related to smaller FSIQ impairment in children admitted after heart- or heart–lung transplantation.
Conclusions
PICU survivors, distinguished in a wide range of subgroups, are at risk of intelligence impairment. Length of PICU stay, female sex and lower rate of survivors were related to greater intelligence impairment. Intelligence outcome has worsened over the years, potentially reflecting the increasing percentage of children surviving PICU admission.
Similar content being viewed by others
Background
Due to major advances in pediatric critical care, the survival rate of children admitted to the pediatric intensive care unit (PICU) has increased dramatically in the past decades [1, 2]. Nevertheless, long-term morbidity after PICU admission is a growing concern [2,3,4,5,6,7,8]. Both the critical illness and the accompanying PICU treatments may impact neurocognitive development as assessed by its gold standard measure intelligence. Intelligence is associated with important life outcomes, such as physical and mental health [9, 10], academic achievement [11], socioeconomic success [12], and life chances in general [10]. These findings highlight intelligence as an important outcome after PICU admission.
Several pathophysiological mechanisms are proposed that may impair long-term intelligence outcome of critically ill patients, including hypoxia, metabolic derangements such as glucose dysregulation, ischemia, inflammation, hypotension and delirium [13,14,15]. These mechanisms may be influenced by the underlying disease [16], critical illness [17] and associated treatments at the PICU [18]. A previous systematic review [19], including 12 studies of which the majority reported on children admitted for sepsis, identified an increased risk of intelligence impairment among PICU survivors. However, meta-analytic quantification of the magnitude of intelligence impairment was not performed, and the available data did not allow to systematically explore predictive factors of intelligence outcome. Given the distinct heterogeneity in the PICU population (e.g. admission indication, associated treatments and age), it is of great importance to determine intelligence outcome of PICU survivors and identify risk factors for poor intelligence outcome.
The current meta-analysis and meta-regression aims to [1] quantify intelligence outcome of PICU survivors; and [2] explore risk factors for poor intelligence outcome. The results of this study will provide valuable information for prognosis and early identification of children at risk for neurocognitive impairment, facilitating determination of the need for follow-up and/or early intervention after PICU discharge.
Methods
Study selection
Inclusion criteria for studies were: [1] the study sample consisted of PICU survivors who had been admitted to a general PICU or specialized PICU; [2] full-scale intelligence quotient (FSIQ) was assessed after PICU hospitalization using (short-forms of) standardized and validated tests; and [3] published in a peer-reviewed journal. Exclusion criteria were: [1] the study reported insufficient data to allow calculation of the individual study’s effect size; [2] the study sample comprised > 5% patients suffering from hereditary syndromes with known intelligence impairment (e.g. Down syndrome); [3] part of the sample comprised children hospitalized at other facilities than the PICU; [4] sample size < 10 children; [5] the study was written in Chinese; [6] the study could not be retrieved via our research institutes or via the authors. In case multiple articles reported on (partly) overlapping cohorts, only one article was selected that reported on (in order of importance): [1] the longest follow-up period; [2] the largest sample size; [3] the most extensive set of risk factors for intelligence impairment.
PubMed, Embase, CINAHL and PsycINFO were searched, without language or date restriction (last search September 7, 2021), using combinations of search terms relating to the [1] PICU, [2] children and [3] intelligence. The complete search strategy is provided in Additional file 1. Studies identified by our search were reviewed by two independent authors and disagreements were solved through discussion or by consulting a third author. Reference lists of the included studies were screened. This meta-analysis was conducted according to PRISMA guidelines.[20] The review protocol was registered in the International Prospective Register of Systematic Reviews, PROSPERO (#CRD42020197282) [21].
Outcomes and covariates
We extracted descriptives on FSIQ of the PICU sample (and healthy control sample, if available) and extracted a broad range of demographic and clinical variables as potential risk factors for poor intelligence outcome. The extracted variables were variables reported at least once in the ten most recently published included articles (see Additional file 1: Table S1). In addition, in articles focusing on cardiac surgery and heart- or heart–lung transplantation, we also extracted the percentage of patients receiving cardiopulmonary bypass (CPB) and/or deep-hypothermic circulatory arrest (DHCA) during surgery, CPB duration during surgery, and the percentage of patients with cyanotic heart disease. To be extracted from an article, the reported risk factor was required to be calculated on at least 75% of the PICU sample. In case only median FSIQ was reported, we calculated the mean [22] and standard deviation (SD) [23]. In case SD of FSIQ was not provided, we used the normative SD (i.e. 15). Two reviewers independently extracted data. Any disagreements were solved through discussion or by consulting a third author.
Study quality
Study quality was assessed using the Newcastle–Ottawa Scale for cohort studies [24]. According to the manual, the scale was adapted to fit the goal of this study (see Additional file 1 for more information [24,25,26]). All included studies were independently rated by two authors and disagreements were solved through discussion or by consulting a third author.
Statistical analysis
Statistical analyses were performed using Comprehensive Meta-Analysis Software version 3.0.[27] For each individual study, FSIQ differences between PICU survivors and either healthy children or normative data were expressed in terms of standardized mean difference scores (Cohen’s d) and used as effect size. In case no healthy control group was included in a study, we used normative data for FSIQ (i.e. mean 100 and SD 15) assuming the same sample size as the PICU sample. Cohen’s d values of 0.2, 0.5 and 0.8, were used to define thresholds for small, medium and large effect sizes, respectively [28].
We calculated a meta-analytic effect size for FSIQ based on all included PICU samples. If a study reported on multiple patient samples separately, one combined effect size was calculated across patient samples before meta-analytic aggregation across studies [29]. In addition, we calculated meta-analytic effect size for a number of PICU subgroup based on the reported indications for PICU admission in the included studies. The available studies allowed to distinguish subgroups of children admitted for: [1] respiratory and/or circulatory insufficiency necessitating ECMO, [2] circulatory insufficiency necessitating CPR [3] traumatic brain injury [4] sepsis and/or meningoencephalitis [5] cardiac surgery [6] heart- or heart–lung transplantation and [7] miscellaneous PICU admission indications. The effect size of each study was weighted by the inverse of its variance to account for sample size and measurement error. Random-effects meta-analysis was performed, recognizing sources of inter-sample variance. Dispersion in effect sizes was quantified using I2, discriminating between mild (I2 < 30), moderate (I2 = 30–50) and strong heterogeneity (I2 > 30) [30]. Indications for publication bias were evaluated using funnel plots and the Egger’s test of asymmetry [31], while the robustness of the meta-analytic effect sizes was calculated by the fail-safe N value, where values > 5 k + 10 were considered robust [32].
In order to determine risk factors for poor intelligence outcome, aggregated effect sizes of PICU subgroups were compared by Cochran’s Q to study whether subgroups differ in the risk for poor intelligence outcome. Subsequently, random-effects meta-regression analyses were performed to quantify the association of each of the demographic and clinical risk factors and the study’s effect sizes for FSIQ. These analyses were performed in the total sample of selected studies and in each PICU subgroup. Meta-regression analyses with < 10 observations were omitted [33].
Results
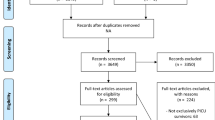
Figure 1 shows the study selection process. Full-text examination revealed 123 eligible studies, published between 1973 and 2021 and comprising 8,119 PICU survivors. Thirty-three studies contained a healthy control group, representing 1,757 healthy control children. Mean year of PICU admission was 2000 (range 1972–2016, k = 99), mean percentage of boys was 59.1% (range 27.0–80.8%, k = 103), mean gestational age was 39.2 weeks (range 35.7–40.6 weeks, k = 46), mean age at PICU admission was 22.4 months (range 0.0–159.6, k = 103), mean time to follow-up was 68.8 months (range 0–231.6, k = 107) and mean age at follow-up was 92.8 months (range 30.1–307.2 months, k = 112). Additional file 1: Table S1 and S2 provide details of all included studies. Inter-rater agreement was 77.4% for study eligibility and 93.3% for quality assessment. The results of quality assessment at the group level are displayed in Fig. 2.
FSIQ
The results of meta-analysis aggregating the results of all 123 studies comparing PICU samples to either healthy controls or normative data (further referred to as controls) are displayed in Fig. 3. The results reveal a small-sized aggregated effect size of d -0.47 (95% CI -0.55 to -0.40, p < 0.001), translating into a FSIQ impairment of on average 7.1 points in PICU survivors. There was strong heterogeneity in the individual study’s effect sizes (I2 = 71.20; p < 0.001).
Risk factors
To study possible sources of the heterogeneity in the individual study’s effect sizes, we analysed subgroups of children based on the reported reasons for PICU admission. All subgroups had significantly lower FSIQ scores than controls (Table 1 and Additional files 2, 3, 4, 5, 6, 7, 8: Figures S1-7). The role of PICU subgroup as risk factor was determined by comparison of the aggregated effect sizes for FSIQ between subgroups. The results indicate that children admitted after heart- or heart–lung transplantation had significantly greater FSIQ impairment (d = -0.80) compared to children admitted after cardiac surgery (d = -0.38, Q = 9.48, p = 0.002) and compared to children admitted for sepsis and/or meningoencephalitis (d = -0.39, Q = 5.85, p = 0.02). Other comparisons between subgroups revealed no significant differences.
The relation between demographic and clinical risk factors and the study’s individual effect sizes for FSIQ was investigated using meta-regression in the total sample (Table 2). Later year of PICU admission was significantly related to greater FSIQ impairment (R2 = 21%, see also Fig. 4), indicating that intelligence outcome of PICU survivors dropped with an average of 2.1 IQ-points every decade between 1972–2016. Furthermore, sex was significantly related to FSIQ (R2 = 5%). This finding indicates that one percentage increase in the percentage of boys in a study was related to an increase of on average 0.1 IQ-points (range studied 27.0–80.8%). In addition, longer PICU staywas significantly related to greater FSIQ impairment (R2 = 2%), indicating that intelligence outcome of PICU survivors dropped with an average of 1.5 IQ-points every additional week of PICU stay (range studied 0.3–35.4 days). Lower rate of survivors (range studied 38.2–100%) was significantly related to greater FSIQ impairment (R2 = 11%), which suggests that survivors in samples with higher mortality have poorer intelligence outcome. Last, higher study quality, as rated on the Newcastle–Ottawa Scale (range 3–7), was significantly related to greater FSIQ impairment (R2 = 7%). No other significant relationships were observed. Of note, no multivariate meta-regression analysis was conducted, because of the low number of studies (k = 29) that reported all risk factors that were found significantly related to FSIQ in the univariate meta-regression analysis, which would lead to biased and underpowered analysis.
Meta-regression in PICU subgroups was possible (i.e. > 10 observations) in the subgroups of children with respiratory and/or circulatory insufficiency necessitating ECMO, cardiac surgery and heart- or heart–lung transplantation (Additional file 1: Table S3). Among children admitted after cardiac surgery, later year of PICU admission (range 1972–2013), lower percentage of boys (range 30.3–79.4%) and higher study quality (range 3–7), were related to greater FSIQ impairment (R2 = 12%, 6% and 9%, respectively). Among children admitted after heart- or heart–lung transplantation, later year of PICU admission (range 1989–2016), younger age at PICU admission (range 1.6–118.4 months) and younger age at follow-up (range 40.7–166.8 months) were related to greater FSIQ impairment (R2 = 65%, 74% and 68%, respectively). None of the other risk factors were related to FSIQ impairment in any of the subgroups.
Publication bias
Inspection of the funnel plot in the total sample did not suggest publication bias (Additional file 9: Figure S8), Egger’s test of asymmetry was not significant (p = 0.50) and the fail-safe N (N = 7,559) indicated that the obtained effect size was robust. Similar results were obtained in the PICU subgroups, with the exception that the fail-safe N values did not support the robustness of the effect sizes obtained in the subgroups of children admitted for circulatory insufficiency necessitating CPR, traumatic brain injury and children with sepsis and/or meningoencephalitis (Table 1).
Use of normative data in uncontrolled studies
We explored the validity of using normative data for the calculation of effect sizes in studies not including a healthy control group. Hence, we calculated effect sizes for studies including a healthy control group (k = 31) with two approaches: [1] using data of the healthy control group and [2] using normative data (i.e. mean 100 and SD 15). Comparisons between the effect sizes retrieved with these two methods revealed a significant difference (Q = 39.5, p < 0.001), with the approach using healthy control group data resulting in a larger aggregated effect size (healthy control group: d = -0.62, 95% CI -0.74 to -0.51, p < 0.001 vs. normative data: d = -0.00, 95% CI -0.16 to 0.16, p = 0.99). This finding was replicated when selecting only those studies with a healthy control group that also tested and confirmed comparability of the PICU and healthy control groups in terms of sex, age and socioeconomic status (most often defined by parental level of education; k = 14; Q = 35.5, p < 0.001).. These findings indicate that the use of normative data yields conservative estimates of FSIQ impairment in PICU survivors.
Discussion
This meta-analysis and meta-regression aimed to [1] quantify intelligence outcome after PICU admission; and [2] explore risk factors for poor intelligence outcome. Based on 123 studies including 8,119 PICU survivors and 1,757 healthy control children, our results demonstrate 0.47 SD lower intelligence scores in PICU survivors compared to controls (healthy control children or normative data), corresponding to an average difference of 7.1 IQ-points. Accordingly, the prevalence of children with intellectual disability (FSIQ < 2 SD [34]) is expected to be threefold higher in PICU survivors (6.4%) than in the general population (2.3%). Intelligence reflects the ability to efficiently process information for goal-directed behavior and is known to be related to physical and mental health [9, 10, 35], academic achievement [11], socioeconomic success [12] and survival to old age [35]. Even a small difference in intelligence can affect profound effects on life chances [10]. These findings highlight the relevance of intelligence outcome and stress the relevance of structured neurocognitive follow-up of PICU survivors.
The results of our study show intelligence impairment across all PICU subgroups investigated, with effect sizes ranging between -0.38 and -0.88 SD. Children admitted after heart- or heart–lung transplantation had significantly greater intelligence impairment (-0.80 SD) compared to children admitted after cardiac surgery (-0.38 SD), and compared to children admitted for sepsis and/or meningoencephalitis (-0.39 SD). This finding may reflect the greater disease severity, greater intensity of PICU treatments, and/or greater intensity of surgical treatment(s) of children admitted after heart- or heart–lung transplantation. The results on the PICU subgroups in the current study are in line with earlier literature overviews [36,37,38,39,40,41,42] and extend these findings by the unique focus on children admitted to the PICU and by providing comprehensive meta-analytic quantification of intelligence impairment.
Meta-regression allowed to study a broad range of demographic and clinical risk factors for intelligence outcome. The results showed that later year of PICU admission (range studied 1972 – 2016) was related to greater intelligence impairment (R2 = 21%). These findings may reflect the increasing medical attainments that have not only led to increased survival rates of children admitted to the PICU, but also to increasing morbidity rates in those surviving [1, 2, 43]. This hypothesis does not find direct support by the contrasting observation that lower rate of survivors (range studied: 38.2–100%) was related to greater intelligence impairment (R2 = 11%). However, differences between survival rate in this analysis may not only reflect potential trends over time, but also differences in the severity of critical illness between conditions that may influence intelligence outcome. In line with this idea, results showed that longer PICU stay (range studied 0.3–35.4 days) was related to greater intelligence impairment (R2 = 2%). This finding may reflect the greater disease severity and/or the greater intensity of PICU treatments of children with longer PICU stay, which may have affected their long-term neurocognitive outcome. Our findings are corroborated by a recent systematic review which also showed that length of PICU stay was related to poorer neurocognitive functioning at discharge [44]. Of note, our current findings indicate that boys had on average better intelligence outcome than girls (R2 = 5%), i.e. every 10 percentage points increase in the amount of boys was related to an increase of on average 1 IQ-point (range studied 27.0–80.8%). No evidence was found for a confounding effect, i.e. girls were not overrepresented in any of the PICU subgroups. The mechanisms underlying sex differences with respect to prevalence and outcome of several neurological conditions are currently not well understood [45]. Sex differences exist in, amongst others, different states in neuroinflammation [45] and (hormonal) reaction to stress [46,47,48]. These sex differences may possibly lead to differences in neurocognitive development of PICU survivors. Understanding the mechanisms behind sex differences could help develop more targeted therapy. At last, meta-regression showed that higher study quality was related to greater intelligence impairment (R2 = 7%). This aligns with the findings of our additional analysis, which showed that the use of the normative mean instead of control group data provides conservative estimates of intelligence impairment.
Regarding subgroups, the meta-regression findings of the total sample were replicated in the subgroup of children admitted after cardiac surgery, with the exception that length of PICU stay was not significantly related to intelligence in this subgroup. Interestingly, longer CPB duration was not related to greater intelligence impairment. This finding contrasts with existing literature from adults showing that CPB duration is related to length of intensive care unit and hospital stay and in-hospital mortality [49]. Taken together, the potential relation between CPB duration and complication risk may not translate into intelligence outcome in children. The results of this study further show that later year of PICU admission was also related to greater intelligence impairment in children with heart- or heart–lung transplantation. In addition, results indicate that younger age at PICU admission was related to greater intelligence impairment in this subgroup. One possible explanation for this finding may be that the main reasons for heart transplantation differ with age (i.e. < 1 year congenital heart disease, > 1 year cardiomyopathy)[50] and congenital heart disease may impact brain development already before birth [51]. We also found that older age at follow-up was associated with smaller intelligence impairment in this subgroup, suggesting that intelligence outcome after heart- or heart–lung transplantation may improve over time.
Intelligence impairment in PICU survivors may be caused by complex interaction between factors related to premorbid functioning [52], underlying disease [53], critical illness [17] and intensive care treatment [54], which influence pathophysiological mechanisms involving (a combination of) hypoxia, metabolic derangements such as glucose dysregulation, ischemia, inflammation, hypotension, delirium and potential negative effects of sedation [13,14,15]. We can only speculate about the specific active (combination) of underlying mechanisms that fuel intelligence impairment in critically ill children admitted to the PICU, which is also likely to differ between subgroups. Nevertheless, our study indicates the need for appropriate prospective studies that provide insight into the potential contribution of pathophysiological mechanisms to intelligence outcome. Such studies may expose potential targets for treatment innovations that may benefit outcome of PICU survivors.
One limitation of this study is that a limited number of possible risk factors was assessed in the included studies (e.g. none of the studies assessed medical history prior to or after PICU admission) and the number of missing data for demographic and clinical potential risk factors was considerable. This reduced the power to identify risk factors (particularly in subgroups). Nevertheless, the available data did allow us to study a broad range of risk factors in the total sample of studies. Furthermore, the current study is limited by the availability of studies into intelligence outcome after PICU admission, with the available studies likely not being fully representative of the typical PICU population in terms of reasons for admission. Our study shows that a substantial number of studies is published mainly on the subgroup of children admitted after cardiac surgery, while other subgroups are less well studied or not at all. For example, we were not able to identify studies including children with respiratory insufficiency necessitating mechanical ventilation or renal insufficiency necessitating renal replacement therapy in our broad and extensive systematic search, while these are important indications for PICU admission [1, 2] and concerns about neurocognitive development of these PICU subgroups exist [4]. This limits the generalizability of our results to the PICU population as a whole and underscores the need for more follow-up studies on these populations. A strength of our study is that with our broad and extensive systematic search we included a considerable number of studies and we were able to aggregate all existing data on intelligence outcome of PICU survivors, to systematically report on subgroups and to comprehensively study risk factors for intelligence impairment. Second, we showed that the use of normative data might underestimate the estimates of intelligence impairment in PICU survivors. Critical appraisal of the role of control data used is important, as normative data are frequently used in research and this may considerably influence the results and conclusions of studies.
Conclusion
In this meta-analysis, robust evidence was found for a risk of intelligence impairment in PICU survivors, applying to a wide range of PICU subgroups. The results further indicate worsening intelligence outcome in the PICU populations over the years (between 1972–2016), potentially reflecting the increasing percentage of children surviving PICU admission with morbidity. In addition, the results indicate that longer length of PICU stay, female sex and lower rate of survivors negatively influence intelligence outcome after PICU admission. The findings of this meta-analysis warrant the need for structured neurocognitive follow-up of PICU survivors.
Availability of data and materials
All data generated or analysed during this study are included in this published article in its supplementary information files.
Abbreviations
- CPB:
-
Cardiopulmonary bypass
- CPR:
-
Cardiopulmonary resuscitation
- DHCA:
-
Deep-hypothermic circulatory arrest
- ECMO:
-
Extra-corporeal membrane oxygenation
- FSIQ:
-
Full-scale intelligence quotient
- PICU:
-
Pediatric intensive care unit
References
Epstein D, Brill JE. A history of pediatric critical care medicine. Pediatr Res. 2005;58(5):987–96.
Namachivayam P, Shann F, Shekerdemian L, Taylor A, van Sloten I, Delzoppo C, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11(5):549–55.
Knoester H, Grootenhuis MA, Bos AP. Outcome of paediatric intensive care survivors. Eur J Pediatr. 2007;166(11):1119–28.
Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr Crit Care Med. 2014;15(7):640–8.
Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15(9):821–7.
Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med. 2017;18(3):e122–30.
Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, et al. Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. J Pediatr. 2018;198:16–24.
Manning JC, Pinto NP, Rennick JE, Colville G, Curley MAQ. Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med. 2018;19(4):298–300.
Koenen KC, Moffitt TE, Roberts AL, Martin LT, Kubzansky L, Harrington H, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166(1):50–7.
Gottfredson LS. Why g Matters: The Complexity of Everyday Life. Intelligence. 1997;24(1):79–132.
Petrill SA, Wilkerson B. Intelligence and Achievement: A Behavioral Genetic Perspective. Educ Psychol Rev. 2000;12(2):185–99.
Strenze T. Intelligence and socioeconomic success: A meta-analytic review of longitudinal research. Intelligence. 2006;35(5):401–26.
Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology. 1992;42(4):733–8.
Johnston MV. Excitotoxicity in perinatal brain injury. Brain Pathol. 2005;15(3):234–40.
Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006;130(3):869–78.
Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153(1):55–60.
Vermunt LC, Buysse CM, Joosten KF, Duivenvoorden HJ, Hazelzet JA, Verhulst FC, et al. Survivors of septic shock caused by Neisseria meningitidis in childhood: psychosocial outcomes in young adulthood. Pediatr Crit Care Med. 2011;12(6):e302–9.
Langenbacher D, Nield T, Poulsen MK. Neurodevelopmental Outcome of ECMO Survivors at Five Years of Age: The Potential for Academic and Motor Difficulties. J Special Educ. 2001;35(3):156–60.
Kachmar AG, Irving SY, Connolly CA, Curley MAQ. A Systematic Review of Risk Factors Associated With Cognitive Impairment After Pediatric Critical Illness. Pediatr Crit Care Med. 2018;19(3):e164–71.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
PROSPERO, International prospective register of systematic reviews. National Institute for Health Research. Available from: https://www.crd.york.ac.uk/PROSPERO.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Eilertsen T, Thorsen AL, Holm SE, Bøe T, Sørensen L, Lundervold AJ. Parental socioeconomic status and child intellectual functioning in a Norwegian sample. Scand J Psychol. 2016;57(5):399–405.
Hanscombe KB, Trzaskowski M, Haworth CM, Davis OS, Dale PS, Plomin R. Socioeconomic status (SES) and children’s intelligence (IQ): in a UK-representative sample SES moderates the environmental, not genetic, effect on IQ. PLoS ONE. 2012;7(2): e30320.
Borenstein M. Comprehensive meta-analysis. Englewood, NJ: Biostat; 2005.
Sullivan GM, Feinn R. Using Effect Size-or Why the P Value Is Not Enough. J Grad Med Educ. 2012;4(3):279–82.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. 2009;77(3):217–24.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Cooper HM, Rosenthal R. Statistical versus traditional procedures for summarizing research findings. Psychol Bull. 1980;87(3):442–9.
EMGO+. Prognostic and Diagnostic Tests. Quality Handbook version 2.0. 2015.
Arlington VA. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Virginia, USA: merican Psychiatric Association; 2013.
Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86(1):130–47.
Schiller RM, Tibboel D. Neurocognitive Outcome After Treatment With(out) ECMO for Neonatal Critical Respiratory or Cardiac Failure. Front Pediatr. 2019;7:494.
Topjian AA, de Caen A, Wainwright MS, Abella BS, Abend NS, Atkins DL, et al. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140(6):e194–233.
Baum M, Freier MC, Chinnock RE. Neurodevelopmental outcome of solid organ transplantation in children. Pediatr Clin North Am. 2003;50(6):1493–503 x.
Alshaikh B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol. 2013;33(7):558–64.
Königs M, Engenhorst PJ, Oosterlaan J. Intelligence after traumatic brain injury: meta-analysis of outcomes and prognosis. Eur J Neurol. 2016;23(1):21–9.
Huisenga D, La Bastide-Van GS, Van Bergen A, Sweeney J, Hadders-Algra M. Developmental outcomes after early surgery for complex congenital heart disease: a systematic review and meta-analysis. Dev Med Child Neurol. 2021;63(1):29–46.
Feldmann M, Bataillard C, Ehrler M, Ullrich C, Knirsch W, Gosteli-Peter MA, et al. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics. 2021;148(4):e2021050875.
Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103(19):2376–81.
Royer AS, Busari JO. A systematic review of the impact of intensive care admissions on post discharge cognition in children. Eur J Pediatr. 2021;180(12):3443–54.
Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–33.
Carpenter T, Grecian SM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8(2):244–55.
Hodes GE, Epperson CN. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol Psychiatry. 2019;86(6):421–32.
Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–82.
Chalmers J, Pullan M, Mediratta N, Poullis M. A need for speed? Bypass time and outcomes after isolated aortic valve replacement surgery. Interact Cardiovasc Thorac Surg. 2014;19(1):21–6.
Boucek MM, Edwards LB, Keck BM, Trulock EP, Taylor DO, Hertz MI. Registry for the International Society for Heart and Lung Transplantation: seventh official pediatric report. J Heart Lung Transplant. 2004;23(8):933–47.
Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25(1):32–6.
Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(s10):2–10.
Donofrio MT, Massaro AN. Impact of congenital heart disease on brain development and neurodevelopmental outcome. Int J Pediatr. 2010;2010:359390.
Madderom MJ, Toussaint L, van der Cammen-van Zijp MH, Gischler SJ, Wijnen RM, Tibboel D, et al. Congenital diaphragmatic hernia with(out) ECMO: impaired development at 8 years. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F316–22.
Acknowledgements
We thank Faridi S. van Etten-Jamaludin, clinical librarian at the Amsterdam UMC, for her help with the search strategy.
Funding
This study was supported by grants of the Janivo, Vaillant and Louise Vehmeijer charity foundations. This study was supported by the Emma’s Children Hospital, Amsterdam UMC. The funder did not participate in the work.
Author information
Authors and Affiliations
Contributions
EdS conceptualized and designed the study, performed study selection and data extraction, carried out the analyses and wrote the manuscript. OvL performed study selection and data extraction, reviewed and revised the manuscript. HK and JvW conceptualized and designed the study, supervised data collection and critically reviewed the manuscript. MK and JO conceptualized and designed the study, supervised data collection and analyses, and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Table S1-S3, additional information on the assessment of the study quality, and the search strategy. Table S1 - Details on PICU groups studied in the included studies, Table S2 - Study characteristics and FSIQ differences (Cohen’s d) between PICU survivors and controls, Table S3 - Results of univariate meta-regression analyses of risk factors for FSIQ impairment in PICU subgroups.
Additional file 2.
Figure S1 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Respiratory and/or circulatory insufficiency necessitating ECMO, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 3.
Figure S2 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Circulatory insufficiency necessitating CPR, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 4.
Figure S3 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Traumatic brain injury, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 5.
Figure S4 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Sepsis and/or meningoencephalitis, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 6.
Figure S5 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Cardiac surgery, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 7.
Figure S6 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Heart- or heart-lung transplantation, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 8.
Figure S7 - Forest plot showing standardized mean differences and accompanying 95% CI of studies reporting on the subgroup Miscellaneous PICU admission indications, comparing FSIQ of PICU survivors to healthy controls or normative data.
Additional file 9.
Figure S8 - Funnel plot of the study’s individual effect sizes for FSIQ plotted against its standard error.
Additional file 10.
References of the studies included in the meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Sonnaville, E.S.V., Kӧnigs, M., van Leijden, O. et al. Intelligence outcome of pediatric intensive care unit survivors: a systematic meta-analysis and meta-regression. BMC Med 20, 198 (2022). https://doi.org/10.1186/s12916-022-02390-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02390-5