Abstract
Objective
Density gradient centrifugation (DGC) is commonly used for sperm preparation before assisted reproductive technology (ART) procedures. This technique separates superior motile spermatozoa with normal morphology from the total sperm population. However, there is still controversy as to the effects of this sperm separation technique on sperm cell DNA integrity which is a determining element in the process of fertilization and embryonic development.
The objective of this study was to determine the effects of DGC on sperm cell DNA integrity as assessed by a novel association between two cytogenetic tests.
Study designs
Semen samples were collected from 30 fertile donors and 40 patients being candidates for ART treatment. Each sample was divided into two parts: the first portion was subjected to selection by two layers of DGC (45% and 90%) and the second fraction was rinsed with phosphate-buffered saline solution and centrifuged without density gradient.
Abnormal sperm chromatin structure as evaluated by a sperm chromatin dispersion (SCD) test and DNA denaturation as assessed by an acridine orange (AO) test were monitored in the initially washed sample and in the different layers of the density gradient centrifugation.
Results
DGC significantly improved the proportion of sperm progressive motility, total motility, and sperm morphology. Moreover, following density gradient centrifugation, the proportion of spermatozoa with denaturated DNA significantly decreased when compared with whole semen (p < 0.001). In addition, we found that spermatozoa isolated in the 90% layer possessed a significantly lower percentage of sperm chromatin decondensation when compared with those remaining in the 45% layer and unprocessed semen (p < 0.001).
Conclusions
Using double cytogenetic tests, our study shows that semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity and recommended to be used in sperm preparation for assisted reproduction.
Similar content being viewed by others
Introduction
Assisted reproductive technology (ART) reports for about five million childbirth in the world from their first clinical application and this number keeps increasing continually [1].
The global decline in sperm parameters worldwide encourages the improvement of a broad range of various laboratory methods concentrating on the separation and enhancement of qualified gametes from the semen [2].
Within in vivo conditions, potentially fertile spermatozoa are isolated from dead or immobile spermatozoa and other cells present in the semen (leukocytes, red blood cells …) in the female genital tract [3]. Over this passage, sustained physiological changes named capacitation also occur which are essential conditions for the sperm’s functional ability to activate acrosome reaction [4, 5].
Currently, sperm selection techniques used for ART, such as density gradient centrifugation (DGC), are trying to mimic the natural separation boundaries and choose sperm selected primarily based on their motility and morphology.
The density gradient technique is based on the centrifugation of semen on different levels of particular solutions with different concentrations. A mature and morphologically normal spermatozoon has a slightly elevated density of 1.10 g/mL. However, an immature and morphologically atypical spermatozoon has a decreased density between 1.06 and 1.09 g/mL [6]. At the end of the process of centrifugation, each cell is situated at the gradient layer appropriate for its density. Thus, the obtained interface between the seminal plasma and different density levels restrain the leukocytes and dead spermatozoa with impaired morphology and/or motility are eliminated. The particularly motile, morphologically normal, and viable spermatozoa are enhanced in the pellet at the bottom of the tube. Therefore, this technique is considered as an ideal sperm treatment that selects a highly functional sperm population [7].
The favorable outcome associated with this technique, however, is trivial maybe since sperm separation is exclusively founded on usual norms such as viability, motility, and morphology [8]. It seems that standard sperm analysis is insufficient because it does not adequately reflect the sperm nuclear DNA and chromatin quality. Those markers have an important part in the process of both natural and in vitro fertilization as well as the development of the subsequent embryo [9].
The nuclear quality of the parental genome concerns, on the one hand, the genetic inheritance transmitted to the conceptus by the spermatozoa and, on the other hand, the chromatin compaction acquired during spermiogenesis, aiming to protect this genetic inheritance during the transit of the male gamete from the seminiferous tube to the oocyte cytoplasm. Hence, any anomalies in chromatin remodeling can engender nuclear damages such as DNA denaturation or fragmentation that could harm male fertility [10, 11]. Besides, previous reports have confirmed that getting a DNA fragmentation index (DFI) more than 30% is related to a poor chance of both natural and assisted reproduction [9,10,11,12].
The question about whether DGC increases or decreases DFI is currently unclear. Indeed, many studies have reported that DGC improves the yield of DNA intact spermatozoa and removes spermatozoa with deficiently condensed chromatin as measured by different sperm DNA integrity techniques [8, 13, 14]. However, others have reported no changes in selecting sperm with high DNA integrity or even the opposite by stipulating that the process of centrifugation itself might cause sperm DNA alterations [15, 16].
Taking into account these contrasting and ambiguous results, we aimed in the current report to examine the efficiency of DGC in enhancing sperm parameters and DNA quality in semen samples of infertile patients using a novel association between two cytogenetic tests, the SCD and AO. Ours is the first report which sheds the light on the impact of DGC in selecting spermatozoa with good quality DNA using two different techniques on each semen sample.
Material and methods
Semen specimens were gathered from 40 men attending the Department of Cytogenetic and Reproductive Biology of Fatuouma Bourguiba University Teaching Hospital (Monastir, Tunisia) before an IVF attempt. Moreover, 30 men with regular semen parameters were chosen as controls.
All of the selected subjects had no history of radiotherapy, chemotherapy, chronic illness, or medication. They were also non-alcoholic and non-smokers. Patients with azoospermia or leucospermia were also excluded from this study.
This protocol was admitted by the local ethics committee and all patients and controls had already given written informed consent.
Semen analysis
Semen specimens were obtained by masturbation into sterile containers, after 2–5 days of sexual abstinence. After 30 min of semen liquefaction at 37°C, standard sperm parameters were analyzed and interpreted according to the World Health Organization (WHO) guidelines [17] and sperm morphology evaluation was performed according to the modified David classification [18].
After semen analysis, samples provided from the 40 patients were fractioned into two similar portions: the first portion was subjected to selection by two layers of DGC and the second fraction was rinsed with phosphate-buffered saline solution (Gibco™ PBS, pH 7.4) and centrifuged at 1100 rpm for 7 min without density gradient. After DGC and the centrifugation of the unprocessed fraction, the two obtained pellets were prepared for AO and SCD techniques.
They were fixed separately in methanol/acetic acid (3:1) and stored at − 20 °C till a later evaluation of sperm DNA denaturation with AO. In order to access DNA condensation with the SCD technique, the second aliquots, from each pellet, were diluted with phosphate buffer saline solution in order to obtain a concentration of 5 to10 millions.
Density gradient centrifugation
The sperm samples were processed using 45% and 90% SpermGradTM gradient layers. Constituents of the density gradient solution contained a colloidal suspension of silica particles adjusted with covalently bonded hydrophilic silane supplied in HEPES. Sperm washing medium (G-IVFTM) was employed to clean and resuspend the final pellet. Gradient medium, sperm wash medium, and semen samples were stored in an incubator at 37°C and 6% CO2 atmosphere for 20 min for equilibration prior to the procedure.
Briefly, 1.5 ml of the lower phase gradient (90%) was moved into a conical bottom tube. A second 1.5-ml layer of the upper phase (45%) was then slowly placed over of the lower phase. A distinct line separating the two layers was observed. A proper volume of liquefied semen was gently placed over of the upper phase. The prepared tube was then centrifuged at 1100 rpm for 10 min.
The supernatant seminal plasma was discarded and every layer of the gradient was gathered aside and washed with 5 ml of RPMI (1640, BioWhittake) at 1100 rpm for 10 min. The sperm pellet acquired after each centrifugation was stored separately.
Acridine orange test (AO)
Spermatozoa, obtained as described above, were brought to room temperature and centrifuged at 400g for 10 min. The pellet was resuspended in 100 μl Tris NaCl EDTA buffer (100 mM NaCl; 50 mM Tris–HCl, pH = 7.4; 0.1 mM EDTA) and kept in ice until staining. The suspension was handled with an acid solution (pH 2.7) (0.2% Triton X-100, 0.2 mol/L NaCl, and 0.08 N/L HCl), maintained for 50 s, and mixed with 2 ml acridine orange solution (1%) in buffer containing 0.2 M citric acid, 0.3 M Na2 HPO4 1 mM EDTA, and 0.15 M, (pH 6.2) NaCl. After 5 min of incubation, samples were centrifuged at 500g for 10 min and pellets were resuspended in 70 μl TNE buffer and then removed to a glass slide. A total of 300 spermatozoa were counted under a fluorescent microscope in 40× magnification, with excitation at 470 nm. Sperm head with intact double-sprigged DNA stained green and those with denatured DNA exhibited red fluorescence [19].
Sperm chromatin dispersion test (SCD)
To assess sperm chromatin integrity, we applied the method described by Fernandez et al. [20]. Shortly, 30 μl of semen samples diluted with phosphate-buffered saline (PBS) was blended with 1% low melting point agarose preserved at 37°C. Approximately, 30 μl of this mixture was pipetted into a pre-coated agarose slide with 0.65% of normal melting point agarose, gently covered with a coverslip, and maintained to solidify for 5 min at 4 °C. The slides were then immersed in freshly prepared acid denaturation solution (0.08 M HCl) for 7 min.
Proteins were eliminated by covering the slides in lysing solution (0.4 M Tris, 0.8 M DTT, 50 mM EDTA, 1% SDS, and 1% Triton X-100) and incubated for 25 min at room temperature. Slides were rinsed with distilled water, pursued by dehydration for 2 min in graded ethanol (70% and 90%), and air dried. Cells were colored with Giemsa and analyzed under a bright-field microscope for halos detection. A minimum of 300 spermatozoa per sample were evaluated by observing the corresponding halo size. Sperm with big- and medium-sized halos were considered normal, and sperm with restricted sized halo or less than one-third of the diameter of the sperm head were considered to have a significant DNA decondensation level.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences, version 18 (SPSS Inc., Chicago, IL, USA). Results are represented as mean ± SD (standard deviation). Differences in the mean values of both DNA decondensation and denaturation between fresh and processed semen for the same patient were made using the ANOVA test.
Pearson’s correlation was performed to examine the relationship between chromatin decondensation and sperm DNA denaturation.
A statistically significant difference was accepted when the p value was < 0.05.
Results
Patient’s characteristics, standard semen analysis, and chromatin status in control and patient groups
The mean age was comparable between patients and controls (33.46 ± 7.25 vs 33 ± 7.54 years, respectively). Sperm parameters of both groups are reported in Table 1. The percentage of progressive motility and abnormal morphology were significantly reduced in patients compared to controls (p < 0.001).
The mean value of DNA denaturation and chromatin decondensation in the initial semen of the forty patients were 49.76 ± 31.56% and 44.77 ± 8.13%, respectively.
Correlation analysis showed a significant positive correlation between sperm denaturation (AO) and decondensation (SCD) rates in the patient group before density gradient centrifugation procedure (r = 0. 897; p < 0.001) (Fig. 1).
Effectiveness of sperm density gradient centrifugation in selecting sperm with better sperm parameters
As presented in Table 2, we noted a significant improvement in both total and progressive sperm motility following density gradient preparation in the patient’s group.
In addition, our results have shown a significant increase in the percentage of spermatozoa with normal morphology and a decrease in the multiple anomalies index value after DGC.
A significant decline in sperm head abnormalities and abnormal acrosome was also noted. Details of morphological abnormalities are shown in Table 2.
Effectiveness of sperm density gradient centrifugation in selecting sperm with lower DNA denaturation and decondensation levels
As shown in Table 3, the level of sperm DNA decondensation was significantly decreased in the 90% fraction as compared to the neat sperm fraction (22.16 ± 6.14% vs 44.77 ± 8.13%, respectively; p < 0.001). The 90% fraction showed also a significant decreased sperm DNA when compared to the 45% fraction (22.16 ± 6.14% vs 45.10 ± 8. 41%, respectively; p < 0.001).
Using the acridine orange test, a significant decreased level of denaturated DNA was shown in the 90% fraction when compared to the initial sperm sample (15.71 ± 10.07% vs 49.76 ± 31.56%, respectively; p < 0.001). The comparison between the two fractions revealed also a significant decreased level of sperm DNA denaturation in the 90% fraction (15.71 ± 10.07% vs 37.12 ± 14.09%, respectively; p < 0.001). There was no statistically significant difference between the 45% fraction and the neat sperm with regard to both DNA fragmentation and denaturation level.
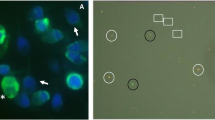
Figure 2 (b and c) shows AO staining in the initial semen sample (Fig. 2b) and after centrifugation in the 90% fraction (Fig. 2c). Figure 2 (d and e) illustrates sperm chromatin structure assessed by the SCD technique test in the initial semen sample (Fig. 2d) and after centrifugation in the 90% fraction (Fig. 2e).
a Percentage of abnormal chromatin structure as assessed by the SCD test and DNA denaturation as evaluated by the AO test in the three spermatic fractions (neat semen, 45% layer, and 90% layer). Sperm DNA denaturation as assessed by acridine orange in the initial semen sample (b) and after centrifugation in the 90% fraction (c). Sperm cell heads with double-stranded DNA were green (AO-green cell); those of single-stranded (denaturated) DNA were red (AO-red cell). Sperm chromatin structure assessed by the SCD technique test in the initial semen sample (d) and after centrifugation in the 90% fraction (e). Sperm cell heads with normal chromatin structure have large halos (Halo +), and those of abnormal chromatin structure have very little or no halos (Halo -)
Discussion
The questionable effectiveness of routine semen examination in establishing male infertility issues has led to the implementation of new techniques allowing the evaluation of sperm DNA integrity and the prediction of fertilization capacity aiming to improve ART outcomes. In fact, evidence suggests that sperm DNA quality adversely affects ART results [21] and fertilization capacity depends on a substantial amount of sperm with chromatin anomalies.
Firstly, our results indicated a significant improvement in the proportion of motile progressive sperm recovered from the 90% layer after the DGC technique which is about 3.5 folds compared with unprocessed semen. This observation consists with those published by Ricci et al. [22], Hashimoto et al. [23], and Noguchi et al. [24] who reported that the DGC technique leads to a higher progressive motile sperm rate than other sperm processing techniques.
Besides, this method revealed its efficiency in the improvement of the proportion of spermatozoa with normal morphology after density gradient sperm preparation as reported in previous studies [21, 25, 26].
In fact, the DGC technique consists of separating the whole semen on layers of particular solutions with different densities. It is a time-saving technique as it only needs around 20 min of centrifugation compared with 1 h of incubation for the swim-up technique. It is simple to perform within sterile conditions which makes it easy to implement in routine clinical work.
Additionally, in the case of a sperm head deformity, the synchronization between head rotation and flagellar motion is altered. Some authors suggest that this desynchronization could explain, among other things, the low fertility potential during conventional in vitro fertilization [27]. This abnormal progression, resulting in variable degrees of asthenospermia, would therefore explain the difficulty faced by these spermatozoa in crossing the two layers of the gradient so that they are not selected. Acrosomal status is a major factor involved in the fertilizing capacity of the sperm as it contains enzymes that are essential to permeate and fertilize the oocyte and take part in capacitation and acrosome reaction [28]. The results of the current study revealed that the number of spermatozoa with normal acrosome was also remarkably increased by 1.4 folds after DGC preparation.
Secondly, using the SCD test, we revealed that spermatozoa retained in the 90% layer after the DGC technique present a significant decrease in the chromatin decondensation rate corresponding to ½ compared to untreated semen. Interestingly, these results are in accordance with those obtained by the AO assay regarding the DNA denaturation levels which decreased from 49.76% in the initial sperm sample to reach a mean value of 15.71% in the 90% layer after centrifugation (p < 0.001). Hence, one of the strengthen points of the current study is that it provides complementary data allowing DNA integrity appreciation: decondensation and denaturation status, especially since these two parameters were evaluated on each semen sample which makes the results more reliable than those where two different techniques were tested but on different semen samples [29].
To go further in selecting the best male germ cells, other reports have proven the efficiency of DGC on isolating the sperm population with longer telomeres [30]. This criterion seems to be predictive on fertilizing sperm ability but currently available data is still controversial [30, 31].
Our results are consistent with other studies focusing on the impact of DGC in reducing the proportion of sperm DNA fragmentation originally present in the neat semen [32, 33].
In accordance with these data, Sakkas et al. [34] noted a significant decrease in the percentage of damaged sperm DNA after DGC, but they did not find any significant amelioration when they used the swim-up method. Moreover, Hammadeh et al. [25] proved that density gradient centrifugation not only ameliorates sperm motility but also leads to the selection of sperm with normal morphology and chromatin integrity.
In a study conducted by Malvezzi et al. [35], three commercially available gradient media (among which SpermGrad that we used in the present study) were employed to evaluate sperm selection quality. Even if conducted on only 20 semen samples, the study has shown that all three media improved sperm parameters as well as DNA quality, which favor previous results that density gradient can recuperate high-quality sperm with little DNA damage. Despite all the previously described trials, the effects of DGC on sperm DNA integrity are still controversial; in fact, other studies have not found DGC to be useful for the selection of sperm with high DNA integrity. A study conducted by Muratori et al. [14], using the pure sperm density gradient, demonstrated that sperm preparation with DGC may even induce sperm DNA fragmentation. They found that after DGC, about 50% of subjects had higher levels of sperm DNA fragmentation when compared to pre-DGC values, proposing the induction of DNA damage over the manipulation. The authors argue for a potential contamination of commercially available colloidal silicon gradients by transition metals which may be the principal reason of sperm DNA breakage during DGC.
This hypothesis was supported by the findings of Aitken et al. [36] who revealed an oxidative DNA damage in sperm following the use of PureSperm® discontinuous colloidal silicon gradients suggesting that metal transition present in the medium particularly Fe, Al, and Cu are responsible to promote free radical generation in the immediate vicinity of DNA. According to these authors, this damage can be significantly accentuated by reducing agents, such as ascorbate (p < 0.001), and inhibited by selective chelation (p < 0.001).
Taken together, it is important that we avow the potential damage that may be induced by certain metals on sperm DNA integrity during sperm preparation and so we must seek to counteract their effects by adding in the medium antioxidants that block the initiation or propagation of chain oxidation. It is well reported that zinc may act by competition with the ferrous ions to the oxygen ligands in the oxidized polyunsaturated fatty acids in the sperm membrane and so it prevents the binding of these redox-active metals [37].
Another study reported by Zini et al. [38] showed a 2-fold augmentation in denatured sperm DNA when compared with raw semen, after processing with two and four layers of Percoll gradients.
The authors attributed this discrepancy to the nature of the gradient itself and the centrifugal force utilized which could be responsible for ROS production and iatrogenic DNA damage induction. The centrifugal force employed when processing the sperm was considerably higher than that used in the current study.
Furthermore, a previous study reported that an excessive amount of ROS produced by seminal leucocytes or immature spermatozoa during sperm incubation has been confirmed to potentially harm sperm function via oxidized DNA adducts and DNA fragmentation [8, 14]. The use of antioxidants in sperm preparation media (albumin was used in this study) can save spermatozoa from oxidative attacks, which could explain the significant amelioration of sperm motility, normal morphology, and especially better DNA quality.
Selecting spermatozoa on the basis of chromatin integrity is a crucial point in the process of fertilization. Indeed, both the lack and the excess of sperm chromatin compaction could be deleterious. On the one hand, a supernormal sperm chromatin compaction could prevent the delivery of the paternal genome in the oocyte [39]. On the other hand, the oocyte has an important DNA repair capacity but is limited to DNA strand breaks and the capacity to manage with an abnormal chromatin structure is very poor [40].
To our knowledge, we herein describe for the first time the relationship between sperm deformity rate and DNA integrity using the SCD and AO assay after DGC preparation.
In fact, we demonstrated a positive correlation between the results provided by these two tests giving us an idea about the sperm DNA integrity by measuring the rate of sperm chromatin dispersion and DNA denaturation.
To conclude, the classic procedures might be ameliorated by conducting in-depth more sophisticated methods, so as to find efficient semen processing that can be used in ART. Therefore, the use of the DGC technique for sperm preparation before ART treatment as reported in this study will be possibly an attractive new strategy that can ameliorate DNA integrity and overcome the shortcomings of routine semen examination to point male infertility issues and to improve ART results.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ART:
-
Assisted reproductive technology
- DGC:
-
Density gradient centrifugation
- SCD:
-
Sperm chromatin dispersion
- AO:
-
Acridine orange
- WHO:
-
World Health Organization
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- RPMI:
-
Roswell Park Memorial Institute
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
References
Adamson M, Tabangin M, Macaluso J, Mouzon D (2013) The number of babies born globally after treatment with the assisted reproductive technologies (ART). Fertil Steril:100–142
KamelR M (2013) Assisted reproductive technology after the birth of Louise Brown. J Reprod Infertil. 14:96–109
Eisenbach M, Giojalas LC (2006) Sperm guidance in mammals: an unpaved road to the egg. Nat Rev Mol Cell Biol. 7:76–85
Mortimer D (1989) Sperm transport in the human female reproductive tract: Oxford reviews of reproductive biology. Oxf-ed, Finn
Rappa KL, Rodriguez HF, Hakkarainen GC, Anchan RM, Mutter GL, Asghar W (2016) Sperm processing for advanced reproductive technologies: where are we today? Biotechnol Adv. 34:578–587
Beydola T, Sharma RK, Agarwal A (2013) Sperm preparation and selection technique. In: Rizk B, Aziz N, Agarwal A, Sabanegh E (eds) Male infertility practice. Jaypee Brothers Medical Publishers, New Delhi
Henkel R, Müller C, Stalf T, Schill WB, Franken DR (1999) Use of failed-fertilized oocytes for diagnostic zona binding purposes after sperm binding improvement with a modified medium. J Assist Reprod Genet. 16:24–29
Jayaraman V, Upadhya D, Narayan PK, Adiga SK (2012) Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 29:557–563
Osman A, Alsomait H, Seshadri S, El-Toukhy T, Khalaf Y (2015) The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 30:120–127
Sakkas D (2013) Novel technologies for selecting the best sperm for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 99:1023–1029
Benchaib M, Braun V, Ressnikof D, Lornage J, Durand P, Niveleau A, Guérin JF (2005) Influence of global sperm DNA methylation on IVF results. Hum Reprod. 20:768–773
González-Marín C, Gosálvez J, Roy R (2012) Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 13:14026–14052
Ahmad L, Jalali S, Shami SA, Akram Z (2007) Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Arch Androl. 53:325–338
Enciso M, Iglesias M, Galán I, Sarasa J, Gosálvez A, Gosálvez J (2011) The ability of sperm selection techniques to remove single- or double-strand DNA damage. Asiatique J Androl. 13:764–768
Zini A, Finelli A, Phang D, Jarvi K (2000) Influence of semen processing technique on human sperm DNA integrity. Urology. 56:1081–1084
Stevanato J, Bertolla RP, Barradas V, Spaine DM, Cedenho AP, Ortiz V (2008) Semen processing by density gradient centrifugation does not improve sperm apoptotic deoxyribonucleic acid fragmentation rates. Fertil Steril. 90:899–890
World Health Organization (2010) Laboratory manual for the examination and processing of human semen, 5th ed. Cambridge University Press, New York
Auger J, Eustache F (2000) Standardization of the morphological assessment of human spermatozoa according to modified David’s classification. Andrologie. 10:358–373
Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 42:87–91
Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG (2003) The sperm chromatin dispersion test: a simple method for the detection of sperm DNA fragmentation. J Androl. 24:59–66
Brahem S, Mehdi M, Elghezal H, Saad A (2011) Semen processing by density gradient centrifugation is useful in selecting sperm with higher double-strand DNA integrity. Andrologia. 43:196–202
Ricci G, Perticarari S, Boscolo R, Montico M, Guaschino S, Presani G (2009) Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrifugation technique. Fertil Steril. 91:632–638
Hashimoto S, Goda S, Akamatsu Y, Yamanaka M, Morimoto Y (2008) Effects of sperm preparation on sperm DNA fragmentation and morphology. Reprod Biomed Online:16–28
Noguchi M, Yoshioka K, Hikono H, Iwagami G, Suzuki C, Kikuchi K (2015) Centrifugation on Percoll density gradient enhances motility, membrane integrity and in vitro fertilizing ability of frozen-thawed boar sperm. Zygote. 23:68–75
Hammadeh ME, Ku¨hnen A, Amer AS, Rosenbaum P, Schmidt W. (2001) Comparison of sperm preparation methods: effect on chromatin and morphology recovery rates and their consequences on the clinical outcome after in vitro fertilization embryo transfer. Int J Androl. 24:360–368
Malvezzi H, Sharma R, Agarwal A, Abuzenadah AM, Abu-Elmagd M (2015) Sperm quality after density gradient centrifugation with three commercially available media: a controlled trial. Reprod Biol Endocrinol. 2:121
Agarwal A, Saleh RA, Bedaiwy MA (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 79:829–843
Karabulut S, Keskin I, Sagıroglu Y (2017) Relationship between semen parameters in samples obtained from sub-fertile patients. Andrology:6–2
Muratori M, Tarozzi N, Carpentiero F, DantiS PM, Cambi M, Casini A, Azzari A, Boni L, Maggi M, Borini A, Baldi E (2019) Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. 16:9–7492
Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, Bukhari I, Xin H, Niu W, Sun Y (2015) Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online:1472–6483
Ghorbani-SiniR IT, Tavalaee M, Azadi L, HajianM ZMR, Nasr-EsfahaniMH. (2020) Comparison of sperm telomere length between two sperm selection procedures: density gradient centrifugation and zeta potential. Int J Fertil Steril. 14:51–56
Wang M, Sun J, Wang L, Gao X, Lu X, Wu Z, Wang Y, Liu K, Tao J, Wu Y (2014) Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet. 31:1655–1663
Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH, Guo BZ, Qin Z (2014) Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet. 31:1161–1166
Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, Bianchi U (2000) The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 15:1112–1116
Malvezzi H, Sharma R, Agarwal A, Abuzenadah AM, Abu-Elmagd M (2014) Sperm quality after density gradient centrifugation with three commercially available media: a controlled trial. Reprod Biol Endocrinol. 12:121
Aitken R, Finnie J, Muscio L, Whiting S, Connaughton H, Kuczer L, Rothkirch T, De Iuliis G (2014) Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod:2136–2147
Zago M, Oteiza P (2001) The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic. Biol. Med:266–274
Zini A, Mak V, Phang D, Jarvi K (1999) Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil Steril. 72:496–499
Björndahl L, Kvist U (2010) Human sperm chromatin stabilization: a proposed model including zinc bridges. Mol Hum Reprod. 16:23–29
Menezo Y, Russo G, Tosti E, Mouatassim S, Benkhalifa M (2007) Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J Assist Reprod Genet. 24:513–520
Acknowledgements
The authors would like to express their gratitude to all the doctors and clinical staff of the Laboratory of Cytogenetics and Reproductive Biology, Fattouma Bourguiba Teaching Hospital, Monastir, Tunisia.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
AssilaHadj Ali, the corresponding author, ensured that the entire group is fully aware of the best practices in the discipline of publication and the full author list and order. Meriem Mehdi finalized the manuscript; in addition to that, all authors have read and approved the final manuscript and contributed substantially in the drafting and revision of the review. Furthermore, all authors have agreed to be personally accountable for the author’s own contributions and that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of Farhat Hached University Teaching Hospital, Sousse, Tunisia.
Besides, all participants signed an informed approval form to participate in this study. The samples were analyzed according to the guidelines and standard procedures of the Center of Maternity and Neonatology, Monastir, Fattouma Bourguiba University Teaching Hospital, Monastir, Tunisia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.H., Ajina, T., Ali, M.B. et al. Efficacy of density gradient centrifugation technique (DGC) in enhancing sperm cell DNA quality for assisted reproductive technique. Middle East Fertil Soc J 27, 22 (2022). https://doi.org/10.1186/s43043-022-00108-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-022-00108-4