Abstract
Purpose
To investigate how effectively density gradient centrifugation (DGC) improves sperm nuclear integrity and to determine whether the sperm chromatin dispersion (SCD) test of sperm nuclear integrity in native or DGC-treated semen can predict the outcome of assisted reproductive technology (ART) in couples undergoing intracytoplasmic sperm injection (ICSI).
Methods
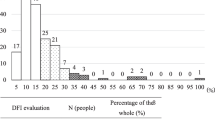
The DNA integrity of spermatozoa from 63 male factor infertility patients undergoing ICSI was analyzed by the SCD test before and after DGC. The predictive value of the sperm DNA fragmentation index (DFI) for ART outcomes was assessed in a cohort of 45 patients who were undergoing fresh embryo transfer. For the analysis, they were divided into pregnant and non-pregnant groups and, independently, into high sperm DFI (DFI > 30 %) and low sperm DFI (DFI ≤ 30 %) groups. Both raw and DGC semen parameters were examined.
Results
In the asthenospermia and oligozoospermia groups, DGC decreased the sperm DFI from 31.5 ± 19.7 and 28.5 ± 10.3 to 19.2 ± 18.3 and 16.0 ± 12.8, respectively (P < 0.01). DGC decreased the sperm DFI in the severe oligozoospermia group from 41.4 ± 19.0 to 36.3 ± 20.6 (P > 0.01). The pregnant and non-pregnant groups did not differ in their fertilization rate and sperm DFI in native or DGC semen (P > 0.05). There was also no significant difference between the high sperm DFI (DFI > 30 %) and low sperm DFI (DFI ≤ 30 %) groups with regard to fertilization rate, implantation rate, and clinical pregnancy rate for both native and DGC semen (P > 0.05). The patients undergoing ICSI with a high sperm DFI had a higher pregnancy loss rate (defined as spontaneous miscarriage or biochemical pregnancy) compared with patients with a low sperm DFI in both the native and DGC semen groups.
Conclusions
DGC highly significantly reduces sperm DNA fragmentation in the semen of ICSI patients, with the exception of those with severe oligozoospermia. The results of the SCD test of sperm DNA fragmentation in native or DGC semen do not correlate with the fertilization rate, implantation rate, or clinical pregnancy rate in patients undergoing ICSI.



Similar content being viewed by others
References
Jackson RE, Bormann CL, Hassun PA, Rocha AM, Motta EL, Serafini PC, et al. Effects of semen storage and separation techniques on sperm DNA fragmentation. Fertil Steril. 2010;94(7):2626–30. doi:10.1016/j.fertnstert.2010.04.049.
Avendano C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94(2):549–57. doi:10.1016/j.fertnstert.2009.02.050.
Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian j of androl. 2008;10(5):786–90. doi:10.1111/j.1745-7262.2008.00417.x.
Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Francois GJ. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87(1):93–100. doi:10.1016/j.fertnstert.2006.05.057.
Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2(8):613–9.
Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56(3):602–7.
Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52(4):864–7.
Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum reprod (Oxf, Engl). 1999;14(4):1039–49.
Chohan KR, Griffin JT, Lafromboise M, De Jonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27(1):53–9. doi:10.2164/jandrol.05068.
Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24(1):59–66.
Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84(4):833–42. doi:10.1016/j.fertnstert.2004.11.089.
Zhang LH, Qiu Y, Wang KH, Wang Q, Tao G, Wang LG. Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nick-end labeling assay. Fertil Steril. 2010;94(3):1027–32. doi:10.1016/j.fertnstert.2009.04.034.
Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15(8):1717–22.
Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, Sakkas D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16(10):2160–5.
Bungum M, Spano M, Humaidan P, Eleuteri P, Rescia M, Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum reprod (Oxf, Engl). 2008;23(1):4–10. doi:10.1093/humrep/dem353.
Jayaraman V, Upadhya D, Narayan PK, Adiga SK. Sperm processing by swim-up and density gradient is effective in elimination of sperm with DNA damage. J Assist Reprod Genet. 2012;29(6):557–63. doi:10.1007/s10815-012-9742-x.
Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20(12):3476–80. doi:10.1093/humrep/dei266.
Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21(11):2876–81. doi:10.1093/humrep/del251.
Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174–9. doi:10.1093/humrep/del326.
Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90(2):352–9. doi:10.1016/j.fertnstert.2007.06.018.
de la Calle JF V, Muller A, Walschaerts M, Clavere JL, Jimenez C, Wittemer C, et al. Sperm deoxyribonucleic acid fragmentation as assessed by the sperm chromatin dispersion test in assisted reproductive technology programs: results of a large prospective multicenter study. Fertil Steril. 2008;90(5):1792–9. doi:10.1016/j.fertnstert.2007.09.021.
Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, Lewis SE. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod. 2010;25(7):1594–608. doi:10.1093/humrep/deq103.
Speyer BE, Pizzey AR, Ranieri M, Joshi R, Delhanty JD, Serhal P. Fall in implantation rates following ICSI with sperm with high DNA fragmentation. Hum Reprod. 2010;25(7):1609–18. doi:10.1093/humrep/deq116.
Lu X, Wu Y, Gao XH, Wang YW, Wang L, Sun XX. Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril. 2012;98(1):131–5. doi:10.1016/j.fertnstert.2012.03.055.
Greenblatt EM, Meriano JS, Casper RF. Type of stimulation protocol affects oocyte maturity, fertilization rate, and cleavage rate after intracytoplasmic sperm injection. Fertil Steril. 1995;64(3):557–63.
Zini A, Mak V, Phang D, Jarvi K. Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil Steril. 1999;72(3):496–9.
Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56(6):1081–4.
Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74(4):824–7.
Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reprod (Camb, Engl). 2001;122(4):497–506.
Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71(1):162–4. doi:10.1086/341096.
Enciso M, Alfarawati S, Wells D. Increased numbers of DNA-damaged spermatozoa in samples presenting an elevated rate of numerical chromosome abnormalities. Hum reprod (Oxf, Engl). 2013;28(6):1707–15. doi:10.1093/humrep/det077.
Meseguer M, Santiso R, Garrido N, Garcia-Herrero S, Remohi J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95(1):124–8. doi:10.1016/j.fertnstert.2010.05.055.
Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum reprod (Oxf, Engl). 2002;17(4):990–8.
Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80(4):895–902.
Huang CC, Lin DP, Tsao HM, Cheng TC, Liu CH, Lee MS. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril. 2005;84(1):130–40. doi:10.1016/j.fertnstert.2004.08.042.
Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril. 2011;95(2):652–7. doi:10.1016/j.fertnstert.2010.08.019.
Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81(5):1289–95. doi:10.1016/j.fertnstert.2003.09.063.
Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril. 2005;84(2):356–64. doi:10.1016/j.fertnstert.2005.02.032.
Tesarik J, Mendoza C, Greco E. Paternal effects acting during the first cell cycle of human preimplantation development after ICSI. Hum Reprod. 2002;17(1):184–9.
Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod biomed online. 2007;14(3):384–95.
Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27(10):2908–17. doi:10.1093/humrep/des261.
Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–61. doi:10.1038/332459a0.
Lewis SE, Boyle PM, McKinney KA, Young IS, Thompson W. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril. 1995;64(4):868–70.
Tremellen K. Oxidative stress and male infertility–a clinical perspective. Hum Reprod Update. 2008;14(3):243–58. doi:10.1093/humupd/dmn004.
Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. The Cochrane database of systematic reviews. 2011(1):CD007411. doi:10.1002/14651858.CD007411.pub2
Acknowledgment
The authors thank International Peace Maternity and Child Health Hospital Affiliated for funding the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Wang and Jian Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, M., Sun, J., Wang, L. et al. Assessment of density gradient centrifugation (DGC) and sperm chromatin dispersion (SCD) measurements in couples with male factor infertility undergoing ICSI. J Assist Reprod Genet 31, 1655–1663 (2014). https://doi.org/10.1007/s10815-014-0339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0339-4